Extracellular Vesicles in Serum and Central Nervous System Tissues Contain microRNA Signatures in Sporadic Amyotrophic Lateral Sclerosis
- PMID: 34776863
- PMCID: PMC8586523
- DOI: 10.3389/fnmol.2021.739016
Extracellular Vesicles in Serum and Central Nervous System Tissues Contain microRNA Signatures in Sporadic Amyotrophic Lateral Sclerosis
Abstract
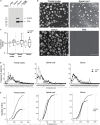
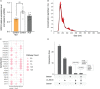
Amyotrophic lateral sclerosis (ALS) is a terminalneurodegenerative disease. Clinical and molecular observations suggest that ALS pathology originates at a single site and spreads in an organized and prion-like manner, possibly driven by extracellular vesicles. Extracellular vesicles (EVs) transfer cargo molecules associated with ALS pathogenesis, such as misfolded and aggregated proteins and dysregulated microRNAs (miRNAs). However, it is poorly understood whether altered levels of circulating extracellular vesicles or their cargo components reflect pathological signatures of the disease. In this study, we used immuno-affinity-based microfluidic technology, electron microscopy, and NanoString miRNA profiling to isolate and characterize extracellular vesicles and their miRNA cargo from frontal cortex, spinal cord, and serum of sporadic ALS (n = 15) and healthy control (n = 16) participants. We found larger extracellular vesicles in ALS spinal cord versus controls and smaller sized vesicles in ALS serum. However, there were no changes in the number of extracellular vesicles between cases and controls across any tissues. Characterization of extracellular vesicle-derived miRNA cargo in ALS compared to controls identified significantly altered miRNA levels in all tissues; miRNAs were reduced in ALS frontal cortex and spinal cord and increased in serum. Two miRNAs were dysregulated in all three tissues: miR-342-3p was increased in ALS, and miR-1254 was reduced in ALS. Additional miRNAs overlapping across two tissues included miR-587, miR-298, miR-4443, and miR-450a-2-3p. Predicted targets and pathways associated with the dysregulated miRNAs across the ALS tissues were associated with common biological pathways altered in neurodegeneration, including axon guidance and long-term potentiation. A predicted target of one identified miRNA (N-deacetylase and N-sulfotransferase 4; NDST4) was likewise dysregulated in an in vitro model of ALS, verifying potential biological relevance. Together, these findings demonstrate that circulating extracellular vesicle miRNA cargo mirror those of the central nervous system disease state in ALS, and thereby offer insight into possible pathogenic factors and diagnostic opportunities.
Keywords: amyotrophic lateral sclerosis; biomarker; central nervous system; extracellular vesicle; microRNA; neurodegeneration; pathway analysis; serum.
Copyright © 2021 Lo, Figueroa-Romero, Hur, Pacut, Stoll, Spring, Lewis, Nair, Goutman, Sakowski, Nagrath and Feldman.
Conflict of interest statement
SG sat on an advisory board for Biogen and IFT Pharma and serves on a Data Safety Monitoring Board. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Andres-Benito P., Moreno J., Aso E., Povedano M., Ferrer I. (2017). Amyotrophic lateral sclerosis, gene deregulation in the anterior horn of the spinal cord and frontal cortex area 8: implications in frontotemporal lobar degeneration. Aging (Albany NY) 9 823–851. 10.18632/aging.101195 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

