PTSD as an Endothelial Disease: Insights From COVID-19
- PMID: 34776871
- PMCID: PMC8586713
- DOI: 10.3389/fncel.2021.770387
PTSD as an Endothelial Disease: Insights From COVID-19
Abstract
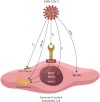
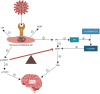
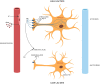
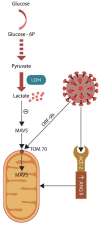
SARS-CoV-2 virus, the etiologic agent of COVID-19, has affected almost every aspect of human life, precipitating stress-related pathology in vulnerable individuals. As the prevalence rate of posttraumatic stress disorder in pandemic survivors exceeds that of the general and special populations, the virus may predispose to this disorder by directly interfering with the stress-processing pathways. The SARS-CoV-2 interactome has identified several antigens that may disrupt the blood-brain-barrier by inducing premature senescence in many cell types, including the cerebral endothelial cells. This enables the stress molecules, including angiotensin II, endothelin-1 and plasminogen activator inhibitor 1, to aberrantly activate the amygdala, hippocampus, and medial prefrontal cortex, increasing the vulnerability to stress related disorders. This is supported by observing the beneficial effects of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in both posttraumatic stress disorder and SARS-CoV-2 critical illness. In this narrative review, we take a closer look at the virus-host dialog and its impact on the renin-angiotensin system, mitochondrial fitness, and brain-derived neurotrophic factor. We discuss the role of furin cleaving site, the fibrinolytic system, and Sigma-1 receptor in the pathogenesis of psychological trauma. In other words, learning from the virus, clarify the molecular underpinnings of stress related disorders, and design better therapies for these conditions. In this context, we emphasize new potential treatments, including furin and bromodomains inhibitors.
Keywords: COVID-19; PTSD; SARS-CoV-2; endothelia; lactate; mitochondria.
Copyright © 2021 Sfera, Osorio, Rahman, Zapata-Martín del Campo, Maldonado, Jafri, Cummings, Maurer and Kozlakidis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

