ROS/JNK/C-Jun Pathway is Involved in Chaetocin Induced Colorectal Cancer Cells Apoptosis and Macrophage Phagocytosis Enhancement
- PMID: 34776955
- PMCID: PMC8578663
- DOI: 10.3389/fphar.2021.729367
ROS/JNK/C-Jun Pathway is Involved in Chaetocin Induced Colorectal Cancer Cells Apoptosis and Macrophage Phagocytosis Enhancement
Abstract
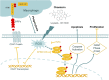
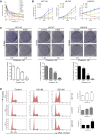
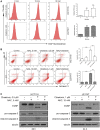
There is an urgent need for novel agents for colorectal cancer (CRC) due to the increasing number of cases and drug-resistance related to current treatments. In this study, we aim to uncover the potential of chaetocin, a natural product, as a chemotherapeutic for CRC treatment. We showed that, regardless of 5-FU-resistance, chaetocin induced proliferation inhibition by causing G2/M phase arrest and caspase-dependent apoptosis in CRC cells. Mechanically, our results indicated that chaetocin could induce reactive oxygen species (ROS) accumulation and activate c-Jun N-terminal kinase (JNK)/c-Jun pathway in CRC cells. This was confirmed by which the JNK inhibitor SP600125 partially rescued CRC cells from chaetocin induced apoptosis and the ROS scavenger N-acetyl-L-cysteine (NAC) reversed both the chaetocin induced apoptosis and the JNK/c-Jun pathway activation. Additionally, this study indicated that chaetocin could down-regulate the expression of CD47 at both mRNA and protein levels, and enhance macrophages phagocytosis of CRC cells. Chaetocin also inhibited tumor growth in CRC xenograft models. In all, our study reveals that chaetocin induces CRC cell apoptosis, irrelevant to 5-FU sensitivity, by causing ROS accumulation and activating JNK/c-Jun, and enhances macrophages phagocytosis, which suggests chaetocin as a candidate for CRC chemotherapy.
Keywords: CD47; JNK/c-Jun pathway; ROS; apoptosis; chaetocin; colorectal cancer.
Copyright © 2021 Wang, Wen, Chen, Li, Qin, He, Wang, Chen, Ye, Li, Peng, Yang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

