Tumor Microenvironment-Responsive Polypeptide Nanogels for Controlled Antitumor Drug Delivery
- PMID: 34776965
- PMCID: PMC8578677
- DOI: 10.3389/fphar.2021.748102
Tumor Microenvironment-Responsive Polypeptide Nanogels for Controlled Antitumor Drug Delivery
Abstract
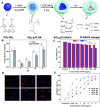
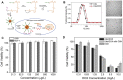
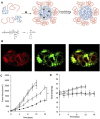
Tumor microenvironment-responsive polypeptide nanogels belong to a biomaterial with excellent biocompatibility, easily adjustable performance, biodegradability, and non-toxic properties. They are developed for selective delivery of antitumor drugs into target organs to promote tumor cell uptake, which has become an effective measure of tumor treatment. Endogenous (such as reduction, reactive oxygen species, pH, and enzyme) and exogenous (such as light and temperature) responsive nanogels can release drugs in response to tumor tissues or cells to improve drug distribution and reduce drug side effects. This article systematically introduces the research progress in tumor microenvironment-responsive polypeptide nanogels to deliver antitumor drugs and provides a reference for the development of antitumor nanoformulations.
Keywords: copolymer; drug delivery; nanogels; nanoparticle; polypeptide; stimulus-responsive.
Copyright © 2021 Liu, Chen, Shi, Zhao and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Abdullah-Al-Nahain N., Nam J. A., Mok H., Lee Y.-k., Park S. Y. (2013). Dual-responsive Crosslinked Pluronic Micelles as a Carrier to Deliver Anticancer Drug Taxol. Macromol. Res. 21 (1), 92–99. 10.1007/s13233-013-1011-z - DOI
-
- Arroyo-Crespo J. J., Armiñán A., Charbonnier D., Balzano-Nogueira L., Huertas-López F., Martí C., et al. (2018). Tumor Microenvironment-Targeted Poly-L-Glutamic Acid-Based Combination Conjugate for Enhanced Triple Negative Breast Cancer Treatment. Biomaterials 186, 8–21. 10.1016/j.biomaterials.2018.09.023 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

