Detection and Monitoring of Mycobacterium leprae Infection in Nine Banded Armadillos (Dasypus novemcinctus) Using a Quantitative Rapid Test
- PMID: 34777319
- PMCID: PMC8581735
- DOI: 10.3389/fmicb.2021.763289
Detection and Monitoring of Mycobacterium leprae Infection in Nine Banded Armadillos (Dasypus novemcinctus) Using a Quantitative Rapid Test
Abstract
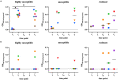
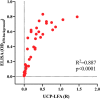
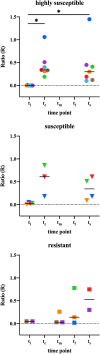
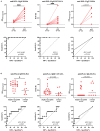
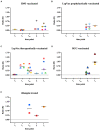
Leprosy is an infectious disease caused by Mycobacterium leprae with tropism for skin and peripheral nerves. Incessant transmission in endemic areas is still impeding elimination of leprosy. Although detection of M. leprae infection remains a challenge in asymptomatic individuals, the presence of antibodies specific for phenolglycolipid-I (PGL-I) correlate with bacterial load. Therefore, serosurveillance utilizing field-friendly tests detecting anti-PGL-I antibodies, can be applied to identify those who may transmit bacteria and to study (reduction of) M. leprae transmission. However, serology based on antibody detection cannot discriminate between past and present M. leprae infection in humans, nor can it detect individuals carrying low bacillary loads. In humans, anti-PGL-I IgM levels are long-lasting and usually detected in more individuals than anti-PGL-I IgG levels. Inherent to the characteristically long incubation time of leprosy, IgM/IgG relations (antibody kinetics) in leprosy patients and infected individuals are not completely clear. To investigate the antibody response directly after infection, we have measured antibody levels by ELISA, in longitudinal samples of experimentally M. leprae infected, susceptible nine-banded armadillos (Dasypus novemcinctus). In addition, we assessed the user- and field-friendly, low-cost lateral flow assay (LFA) utilizing upconverting reporter particles (UCP), developed for quantitative detection of human anti-PGL-I IgM (UCP-LFA), to detect treatment- or vaccination-induced changes in viable bacterial load. Our results show that serum levels of anti-PGL-I IgM, and to a lesser extent IgG, significantly increase soon after experimental M. leprae infection in armadillos. In view of leprosy phenotypes in armadillos, this animal model can provide useful insight into antibody kinetics in early infection in the various spectral forms of human leprosy. The UCP-LFA for quantitative detection of anti-PGL-I IgM allows monitoring the efficacy of vaccination and rifampin-treatment in the armadillo leprosy model, thereby providing a convenient tool to evaluate the effects of drugs and vaccines and new diagnostics.
Keywords: M. leprae; PGL-I; POC; antibodies; armadillo; diagnosis; lateral flow assay; leprosy.
Copyright © 2021 Zhou, Pena, van Hooij, Pierneef, de Jong, Stevenson, Walley, Corstjens, Truman, Adams and Geluk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer WL declared a past collaboration with one of the author MP to the handling editor.
Figures

Similar articles
-
Rapid test for Mycobacterium leprae infection: a practical tool for leprosy.Infect Dis Poverty. 2024 Dec 2;13(1):88. doi: 10.1186/s40249-024-01262-9. Infect Dis Poverty. 2024. PMID: 39617937 Free PMC article.
-
Evidence of zoonotic leprosy in Pará, Brazilian Amazon, and risks associated with human contact or consumption of armadillos.PLoS Negl Trop Dis. 2018 Jun 28;12(6):e0006532. doi: 10.1371/journal.pntd.0006532. eCollection 2018 Jun. PLoS Negl Trop Dis. 2018. PMID: 29953440 Free PMC article.
-
Evaluation of the origin of Mycobacterium leprae infections in the wild armadillo, Dasypus novemcinctus.Am J Trop Med Hyg. 1986 May;35(3):588-93. doi: 10.4269/ajtmh.1986.35.588. Am J Trop Med Hyg. 1986. PMID: 3518509
-
Detection of anti-M. leprae antibodies in children in leprosy-endemic areas: A systematic review.PLoS Negl Trop Dis. 2021 Aug 27;15(8):e0009667. doi: 10.1371/journal.pntd.0009667. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34449763 Free PMC article.
-
Reservoirs and transmission routes of leprosy; A systematic review.PLoS Negl Trop Dis. 2020 Apr 27;14(4):e0008276. doi: 10.1371/journal.pntd.0008276. eCollection 2020 Apr. PLoS Negl Trop Dis. 2020. PMID: 32339201 Free PMC article.
Cited by
-
The Armadillo as a Model for Leprosy Nerve Function Impairment: Preventative and Therapeutic Interventions.Front Med (Lausanne). 2022 Jun 23;9:879097. doi: 10.3389/fmed.2022.879097. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35814754 Free PMC article.
-
Anti-phenolic glycolipid antibodies in Mycobacterium bovis infected cattle.One Health. 2025 Jan 28;20:100982. doi: 10.1016/j.onehlt.2025.100982. eCollection 2025 Jun. One Health. 2025. PMID: 39974705 Free PMC article.
-
Integrative immune analysis in patients with leprosy reveals host factors associated with mycobacterial control.EBioMedicine. 2025 Aug;118:105855. doi: 10.1016/j.ebiom.2025.105855. Epub 2025 Jul 18. EBioMedicine. 2025. PMID: 40683203 Free PMC article.
-
Development of lateral flow assays to detect host proteins in cattle for improved diagnosis of bovine tuberculosis.Front Vet Sci. 2023 Aug 15;10:1193332. doi: 10.3389/fvets.2023.1193332. eCollection 2023. Front Vet Sci. 2023. PMID: 37655261 Free PMC article.
-
Molecular and Serological Surveillance for Mycobacterium leprae and Mycobacterium lepromatosis in Wild Red Squirrels (Sciurus vulgaris) from Scotland and Northern England.Animals (Basel). 2024 Jul 7;14(13):2005. doi: 10.3390/ani14132005. Animals (Basel). 2024. PMID: 38998117 Free PMC article.
References
-
- Bobosha K., Fat E., van den Eeden S. J. F., Bekele Y., van der Ploeg-van, Schip J. J., et al. (2014). Field-evaluation of a new lateral flow assay for detection of cellular and humoral immunity against Mycobacterium leprae. PLoS Negl. Trop. Dis. 8:5. 10.1371/journal.pntd.0002845 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

