Safety of Influenza A H1N1pdm09 Vaccines: An Overview of Systematic Reviews
- PMID: 34777351
- PMCID: PMC8581668
- DOI: 10.3389/fimmu.2021.740048
Safety of Influenza A H1N1pdm09 Vaccines: An Overview of Systematic Reviews
Abstract
Background: In 2009, a new influenza A H1N1 virus emerged causing a global pandemic. A range of monovalent influenza A H1N1pdm09 vaccines with or without adjuvants were developed. After the mass vaccination campaigns safety concerns related to H1N1pdm09 vaccines were reported. More than a decade later, reported AEFIs are still under scrutiny. We performed a systematic review aiming to synthesize the evidence on the safety of the H1N1pdm09 vaccines on reported outcomes from existing systematic reviews.
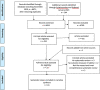
Methods: Four electronic databases, PubMed, EMBASE, Epistimonikos and the Cochrane Database of Systematic Reviews were searched for articles on H1N1pdm09 vaccination published from 2009 to January 2021. Systematic reviews assessing short- or long-term adverse events after H1N1pdm09 vaccination were considered for inclusion. Data was extracted from all selected reviews. Outcomes were grouped and results from each included review were presented narratively and in tables.
Results: 16 systematic reviews met the inclusion criteria. Reported outcomes were short-term events (3 reviews), fetal/pregnancy outcomes (8 reviews), Guillain-Barré syndrome (GBS) (4 reviews), narcolepsy (2 reviews) demyelinating diseases (1 review based on one study only) and inflammatory bowel disease (IBD) (1 review). Short-term serious adverse events were rare, 3 cases amongst 16725 subjects in 18 randomized controlled trials (0.018%). No deaths were reported. The risks of local events were generally higher for adjuvanted vaccines as compared to unadjuvanted vaccines. Maternal H1N1pdm09 vaccination in any trimester was not associated with an increase in preterm birth, small for gestational age, congenital malformations or fetal death. For GBS, results were conflicting. The main systematic review on narcolepsy found a 5-14-fold increased risk in children, and a 2-7- fold increased risk in adults after vaccination with Pandemrix. The attributable risk of narcolepsy one year after vaccination was 1 case per 18 400 vaccine doses in children/adolescents, and 1 case per 181 000 vaccine doses in adults.
Conclusion: Adjuvanted vaccines had more local but not serious adverse events compared to unadjuvanted vaccines. Vaccination with Pandemrix was strongly associated with narcolepsy, particularly in children. No increased risks of pregnancy outcomes were seen after pandemic vaccination. The findings on GBS were inconclusive.
Keywords: H1N1pdm09 vaccination; adverse events; influenza vaccines; pandemic vaccines; safety.
Copyright © 2021 Juvet, Robertson, Laake, Mjaaland and Trogstad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- 2009 H1N1 Pandemic (H1N1pdm09 virus) . Centers for Disease Control and Prevention (CDC) . Available at: https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html (Accessed June 30, 2021).
-
- WHO . New Data on Narcolepsy Following the 2009 Pandemic Influenza Vaccine, in: The Global Advisory Comittee of Vaccine Safety (2017). Available at: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/t... (Accessed June 30, 2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous