Adeno-Associated Viruses (AAV) and Host Immunity - A Race Between the Hare and the Hedgehog
- PMID: 34777364
- PMCID: PMC8586419
- DOI: 10.3389/fimmu.2021.753467
Adeno-Associated Viruses (AAV) and Host Immunity - A Race Between the Hare and the Hedgehog
Abstract
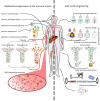
Adeno-associated viruses (AAV) have emerged as the lead vector in clinical trials and form the basis for several approved gene therapies for human diseases, mainly owing to their ability to sustain robust and long-term in vivo transgene expression, their amenability to genetic engineering of cargo and capsid, as well as their moderate toxicity and immunogenicity. Still, recent reports of fatalities in a clinical trial for a neuromuscular disease, although linked to an exceptionally high vector dose, have raised new caution about the safety of recombinant AAVs. Moreover, concerns linger about the presence of pre-existing anti-AAV antibodies in the human population, which precludes a significant percentage of patients from receiving, and benefitting from, AAV gene therapies. These concerns are exacerbated by observations of cellular immune responses and other adverse events, including detrimental off-target transgene expression in dorsal root ganglia. Here, we provide an update on our knowledge of the immunological and molecular race between AAV (the "hedgehog") and its human host (the "hare"), together with a compendium of state-of-the-art technologies which provide an advantage to AAV and which, thus, promise safer and more broadly applicable AAV gene therapies in the future.
Keywords: AAV; antibody response; capsid; cellular response; engineering; immune evasion; neutralizing antibodies; pre-existing immunity.
Copyright © 2021 Rapti and Grimm.
Conflict of interest statement
DG is a co-founder and shareholder (CSO) of AaviGen GmbH. DG and KR are inventors on a pending patent application related to the generation of immune-evading AAV capsid variants.
Figures

Similar articles
-
Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy.Hum Gene Ther Methods. 2013 Apr;24(2):59-67. doi: 10.1089/hgtb.2012.243. Epub 2013 Apr 3. Hum Gene Ther Methods. 2013. PMID: 23442094 Free PMC article. Review.
-
AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer.Mol Ther. 2020 Mar 4;28(3):723-746. doi: 10.1016/j.ymthe.2019.12.010. Epub 2020 Jan 10. Mol Ther. 2020. PMID: 31972133 Free PMC article. Review.
-
Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion.Proc Natl Acad Sci U S A. 2017 Jun 13;114(24):E4812-E4821. doi: 10.1073/pnas.1704766114. Epub 2017 May 30. Proc Natl Acad Sci U S A. 2017. PMID: 28559317 Free PMC article.
-
Immunity to adeno-associated virus vectors in animals and humans: a continued challenge.Gene Ther. 2008 Jun;15(11):808-16. doi: 10.1038/gt.2008.54. Epub 2008 Apr 3. Gene Ther. 2008. PMID: 18385765 Review.
-
Long-Term Follow-Up of the First in Human Intravascular Delivery of AAV for Gene Transfer: AAV2-hFIX16 for Severe Hemophilia B.Mol Ther. 2020 Sep 2;28(9):2073-2082. doi: 10.1016/j.ymthe.2020.06.001. Epub 2020 Jun 10. Mol Ther. 2020. PMID: 32559433 Free PMC article. Clinical Trial.
Cited by
-
Fantastic AAV Gene Therapy Vectors and How to Find Them-Random Diversification, Rational Design and Machine Learning.Pathogens. 2022 Jul 3;11(7):756. doi: 10.3390/pathogens11070756. Pathogens. 2022. PMID: 35890005 Free PMC article. Review.
-
mLumiOpto Is a Mitochondrial-Targeted Gene Therapy for Treating Cancer.Cancer Res. 2024 Dec 2;84(23):4049-4065. doi: 10.1158/0008-5472.CAN-24-0984. Cancer Res. 2024. PMID: 39288077 Free PMC article.
-
Rapid Development of Small Rodent Animal Models for Infectious Disease Research Through Vectorized Receptor Molecule Expression.Viruses. 2024 Nov 19;16(11):1794. doi: 10.3390/v16111794. Viruses. 2024. PMID: 39599908 Free PMC article. Review.
-
Specific detection of duck adeno-associated virus using a TaqMan-based real-time PCR assay.Front Vet Sci. 2024 Nov 13;11:1483990. doi: 10.3389/fvets.2024.1483990. eCollection 2024. Front Vet Sci. 2024. PMID: 39606664 Free PMC article.
-
Genetically engineered mesenchymal stem cells with dopamine synthesis for Parkinson's disease in animal models.NPJ Parkinsons Dis. 2022 Dec 22;8(1):175. doi: 10.1038/s41531-022-00440-6. NPJ Parkinsons Dis. 2022. PMID: 36550118 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

