A Novel Missense Variant of HOXD13 Caused Atypical Synpolydactyly by Impairing the Downstream Gene Expression and Literature Review for Genotype-Phenotype Correlations
- PMID: 34777468
- PMCID: PMC8579070
- DOI: 10.3389/fgene.2021.731278
A Novel Missense Variant of HOXD13 Caused Atypical Synpolydactyly by Impairing the Downstream Gene Expression and Literature Review for Genotype-Phenotype Correlations
Abstract
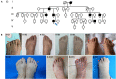
Synpolydactyly (SPD) is a hereditary congenital limb malformation with distinct syndactyly designated as SPD1, SPD2, and SPD3. SPD1 is caused by mutations of HOXD13, which is a homeobox transcription factor crucial for limb development. More than 143 SPD patients have been reported to carry HOXD13 mutations, but there is a lack of genotype-phenotype correlation. We report a novel missense mutation of c. 925A > T (p.I309F) in an individual with atypical synpolydactyly inherited from her father with mild clinodactyly and three other different alanine insertion mutations in HOXD13 identified by whole exome sequencing (WES) in 12 Chinese SPD families. Unlike polyalanine extension, which tends to form α-helix and causes protein aggregation in the cytoplasm as shown by molecular simulation and immunofluorescence, the c. 925A > T mutation impairs downstream transcription of EPHA7. We compiled literature findings and analyzed genotype-phenotype features in 173 SPD individuals of 53 families, including 12 newly identified families. Among the HOXD13-related individuals, mutations were distributed in three regions: polyalanine, homeobox, and non-homeobox. Polyalanine extension was the most common variant (45%), followed by missense mutations (32%) mostly in the homeobox compared with the loss-of-function (LOF) variants more likely in non-homeobox. Furthermore, a more severe degree and classic SPD were associated with polyalanine mutations although missense variants were associated with brachydactyly and syndactyly in hands and feet and LOF variants with clinodactyly in hands. Our study broadens the HOXD13 mutation spectrum and reveals the profile of three different variants and their severity of SPD, the genotype-phenotype correlation related to the HOXD13 mutation site provides clinical insight, including for genetic counseling.
Keywords: HoxD13; genotype and phenotype; polyalanine extension; synpolydactyly; transcription regulation.
Copyright © 2021 Guo, Fang, Mao, Sun, Zhou, An and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer TY declared a shared affiliation with several of the authors, RG, JZ, XF, BS, and BW, to the handling editor at the time of review.
Figures




References
-
- Al-Qattan M. M. (2020). A Review of the Phenotype of Synpolydactyly Type 1 in Homozygous Patients: Defining the Relatively Long and Medially Deviated Big Toe with/without Cupping of the Forefoot as a Pathognomonic Feature in the Phenotype. BioMed. Res. Int. 2020:2067186. 10.1155/2020/2067186 - DOI - PMC - PubMed
-
- Boris U., Karl B., Detlef B., Lentze M. J., Frank B., Michael L. (2002). A novel stable polyalanine [poly(A)] expansion in the HOXA13 gene associated with hand-foot-genital syndrome: proper function of poly(A)-harbouring transcription factors depends on a critical repeat length? Hum. Genet. 110:488. 10.1007/s00439-002-0712-8 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

