Striated muscle proteins are regulated both by mechanical deformation and by chemical post-translational modification
- PMID: 34777614
- PMCID: PMC8555064
- DOI: 10.1007/s12551-021-00835-4
Striated muscle proteins are regulated both by mechanical deformation and by chemical post-translational modification
Abstract
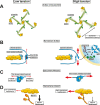
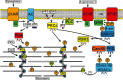
All cells sense force and build their cytoskeleton to optimize function. How is this achieved? Two major systems are involved. The first is that load deforms specific protein structures in a proportional and orientation-dependent manner. The second is post-translational modification of proteins as a consequence of signaling pathway activation. These two processes work together in a complex way so that local subcellular assembly as well as overall cell function are controlled. This review discusses many cell types but focuses on striated muscle. Detailed information is provided on how load deforms the structure of proteins in the focal adhesions and filaments, using α-actinin, vinculin, talin, focal adhesion kinase, LIM domain-containing proteins, filamin, myosin, titin, and telethonin as examples. Second messenger signals arising from external triggers are distributed throughout the cell causing post-translational or chemical modifications of protein structures, with the actin capping protein CapZ and troponin as examples. There are numerous unanswered questions of how mechanical and chemical signals are integrated by muscle proteins to regulate sarcomere structure and function yet to be studied. Therefore, more research is needed to see how external triggers are integrated with local tension generated within the cell. Nonetheless, maintenance of tension in the sarcomere is the essential and dominant mechanism, leading to the well-known phrase in exercise physiology: "use it or lose it."
Keywords: Adhesome; Costamere; Integrin; Mechanobiology; Mechanotransduction; Sarcomere.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2021.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures


Similar articles
-
Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage.J Muscle Res Cell Motil. 2021 Jun;42(2):367-380. doi: 10.1007/s10974-021-09596-9. Epub 2021 Feb 17. J Muscle Res Cell Motil. 2021. PMID: 33595762 Free PMC article.
-
The costamere bridges sarcomeres to the sarcolemma in striated muscle.Prog Pediatr Cardiol. 2011 May;31(2):83-88. doi: 10.1016/j.ppedcard.2011.02.003. Prog Pediatr Cardiol. 2011. PMID: 24039381 Free PMC article.
-
Mechanobiology in cardiac mechanics.Biophys Rev. 2021 Aug 27;13(5):583-585. doi: 10.1007/s12551-021-00827-4. eCollection 2021 Oct. Biophys Rev. 2021. PMID: 34765042 Free PMC article.
-
Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions.Eur J Cell Biol. 2013 Oct-Nov;92(10-11):339-48. doi: 10.1016/j.ejcb.2013.10.009. Epub 2013 Nov 4. Eur J Cell Biol. 2013. PMID: 24252517 Review.
-
Integrin-mediated cell adhesion: the cytoskeletal connection.Biochem Soc Symp. 1999;65:79-99. Biochem Soc Symp. 1999. PMID: 10320934 Review.
Cited by
-
An Atypical F-Actin Capping Protein Modulates Cytoskeleton Behaviors Crucial for Trichomonas vaginalis Colonization.Microbiol Spectr. 2023 Aug 17;11(4):e0059623. doi: 10.1128/spectrum.00596-23. Epub 2023 Jun 13. Microbiol Spectr. 2023. PMID: 37310229 Free PMC article.
-
Transthyretin deposition alters cardiomyocyte sarcomeric architecture, calcium transients, and contractile force.Physiol Rep. 2022 Mar;10(5):e15207. doi: 10.14814/phy2.15207. Physiol Rep. 2022. PMID: 35262277 Free PMC article.
-
Cardiovascular mechanobiology-a Special Issue to look at the state of the art and the newest insights into the role of mechanical forces in cardiovascular development, physiology and disease.Biophys Rev. 2021 Sep 11;13(5):575-577. doi: 10.1007/s12551-021-00842-5. eCollection 2021 Oct. Biophys Rev. 2021. PMID: 34777612 Free PMC article.
-
Cardiac CapZ Regulation During Acute Exercise in Female Mice.FASEB J. 2025 Aug 31;39(16):e70950. doi: 10.1096/fj.202502431R. FASEB J. 2025. PMID: 40832763 Free PMC article.
-
Cardiomyocyte external mechanical unloading activates modifications of α-actinin differently from sarcomere-originated unloading.FEBS J. 2023 Nov;290(22):5322-5339. doi: 10.1111/febs.16925. Epub 2023 Aug 17. FEBS J. 2023. PMID: 37551968 Free PMC article.
References
-
- Ait-Mou Y, Hsu K, Farman GP, Kumar M, Greaser ML, Irving TC, de Tombe PP. Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc Natl Acad Sci U S A. 2016;113:2306–2311. doi: 10.1073/pnas.1516732113. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

