What keeps us ticking? Sinoatrial node mechano-sensitivity: the grandfather clock of cardiac rhythm
- PMID: 34777615
- PMCID: PMC8555057
- DOI: 10.1007/s12551-021-00831-8
What keeps us ticking? Sinoatrial node mechano-sensitivity: the grandfather clock of cardiac rhythm
Abstract
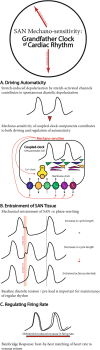
The rhythmic and spontaneously generated electrical excitation that triggers the heartbeat originates in the sinoatrial node (SAN). SAN automaticity has been thoroughly investigated, which has uncovered fundamental mechanisms involved in cardiac pacemaking that are generally categorised into two interacting and overlapping systems: the 'membrane' and 'Ca2+ clock'. The principal focus of research has been on these two systems of oscillators, which have been studied primarily in single cells and isolated tissue, experimental preparations that do not consider mechanical factors present in the whole heart. SAN mechano-sensitivity has long been known to be a contributor to SAN pacemaking-both as a driver and regulator of automaticity-but its essential nature has been underappreciated. In this review, following a description of the traditional 'clocks' of SAN automaticity, we describe mechanisms of SAN mechano-sensitivity and its vital role for SAN function, making the argument that the 'mechanics oscillator' is, in fact, the 'grandfather clock' of cardiac rhythm.
Keywords: Calcium clock; Heart rate; Mechano-electric coupling; Membrane clock; Pacemaking; Stretch.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2021.
Figures



References
-
- Abramovich-Sivan S, Akselrod S (1999) Phase response curve based model of the SA node: simulation by two-dimensional array of pacemaker cells with randomly distributed cycle lengths. Med Biol Eng Comput 37:482–491. 10.1007/BF02513334 - PubMed
-
- Anumonwo JMB, Delmar M, Vinet A, Michfaels DC, Jalife J (1991) Phase resetting and entrainment of pacemaker activity in single sinus nodal cells. Circ Res 68:1138–1153. 10.1161/01.res.68.4.1138 - PubMed
-
- Arai A, Kodama I, Toyama J (1996) Roles of Cl- channels and Ca2+ mobilization in stretch-induced increase of SA node pacemaker activity. Am J Physiol Heart Circ Physiol 270:H1726–H1735. 10.1152/ajpheart.1996.270.5.H1726 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

