Flash chemistry enables high productivity metalation-substitution of 5-alkyltetrazoles
- PMID: 34777760
- PMCID: PMC8528014
- DOI: 10.1039/d1sc04176b
Flash chemistry enables high productivity metalation-substitution of 5-alkyltetrazoles
Abstract
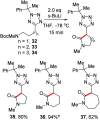
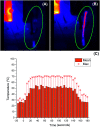
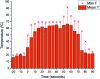
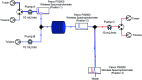
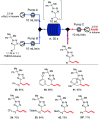
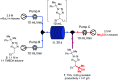
Tetrazoles play a prominent role in medicinal chemistry due to their role as carboxylate bioisosteres but have largely been overlooked as C-H functionalisation substrates. We herein report the development of a high-yielding and general procedure for the heterobenzylic C-H functionalisation of 5-alkyltetrazoles in up to 97% yield under batch conditions using a metalation/electrophilic trapping strategy. Through the use of thermal imaging to identify potentially unsafe exotherms, a continuous flow procedure using a flash chemistry strategy has also been developed, allowing products to be accessed in up to 95% yield. This enabled an extremely high productivity rate of 141 g h-1 to be achieved on an entry-level flow system.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






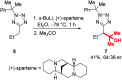


Similar articles
-
Lithiation Substitution of Unprotected Benzyltetrazoles.Org Lett. 2019 Sep 6;21(17):7069-7072. doi: 10.1021/acs.orglett.9b02633. Epub 2019 Aug 26. Org Lett. 2019. PMID: 31449424
-
Batch Versus Flow Lithiation-Substitution of 1,3,4-Oxadiazoles: Exploitation of Unstable Intermediates Using Flow Chemistry.Chemistry. 2019 Sep 20;25(53):12439-12445. doi: 10.1002/chem.201902917. Epub 2019 Aug 29. Chemistry. 2019. PMID: 31361052
-
Main group metal-mediated strategies for C-H and C-F bond activation and functionalisation of fluoroarenes.Chem Sci. 2023 Sep 13;14(42):11617-11628. doi: 10.1039/d3sc03548d. eCollection 2023 Nov 1. Chem Sci. 2023. PMID: 37920337 Free PMC article. Review.
-
Utilising Sodium-Mediated Ferration for Regioselective Functionalisation of Fluoroarenes via C-H and C-F Bond Activations.Angew Chem Int Ed Engl. 2018 Jan 2;57(1):187-191. doi: 10.1002/anie.201709750. Epub 2017 Nov 30. Angew Chem Int Ed Engl. 2018. PMID: 29068502
-
Applications of organocatalysed visible-light photoredox reactions for medicinal chemistry.Beilstein J Org Chem. 2018 Aug 3;14:2035-2064. doi: 10.3762/bjoc.14.179. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30202458 Free PMC article. Review.
Cited by
-
Taming Highly Unstable Radical Anions and 1,4-Organodilithiums by Flow Microreactors: Controlled Reductive Dimerization of Styrenes.JACS Au. 2022 Oct 31;2(11):2514-2521. doi: 10.1021/jacsau.2c00375. eCollection 2022 Nov 28. JACS Au. 2022. PMID: 36465543 Free PMC article.
References
-
- Herr R. J. Bioorg. Med. Chem. 2002;10:3379. - PubMed
-
- Kaczmarek J. Smagowski H. Grzonka Z. J. Chem. Soc., Perkin Trans. 2. 1979:1670. doi: 10.1039/P29790001670. - DOI
-
- Larsen R. D. King A. O. Chen C. Y. Corley E. G. Foster B. S. Roberts F. E. Yang C. Lieberman D. R. Reamer R. A. Tschaen D. M. Verhoeven T. R. Reider P. J. Lo Y. S. Rossano L. T. Brookes A. S. Meloni D. Moore J. R. Arnett J. F. J. Org. Chem. 1994;59:6391. doi: 10.1021/jo00100a048. - DOI
LinkOut - more resources
Full Text Sources

