Flash chemistry enables high productivity metalation-substitution of 5-alkyltetrazoles
- PMID: 34777760
- PMCID: PMC8528014
- DOI: 10.1039/d1sc04176b
Flash chemistry enables high productivity metalation-substitution of 5-alkyltetrazoles
Abstract
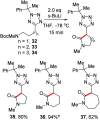
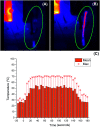
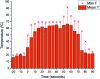
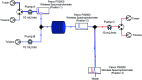
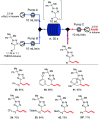
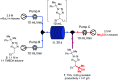
Tetrazoles play a prominent role in medicinal chemistry due to their role as carboxylate bioisosteres but have largely been overlooked as C-H functionalisation substrates. We herein report the development of a high-yielding and general procedure for the heterobenzylic C-H functionalisation of 5-alkyltetrazoles in up to 97% yield under batch conditions using a metalation/electrophilic trapping strategy. Through the use of thermal imaging to identify potentially unsafe exotherms, a continuous flow procedure using a flash chemistry strategy has also been developed, allowing products to be accessed in up to 95% yield. This enabled an extremely high productivity rate of 141 g h-1 to be achieved on an entry-level flow system.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






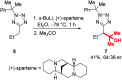


References
-
- Herr R. J. Bioorg. Med. Chem. 2002;10:3379. - PubMed
-
- Kaczmarek J. Smagowski H. Grzonka Z. J. Chem. Soc., Perkin Trans. 2. 1979:1670. doi: 10.1039/P29790001670. - DOI
-
- Larsen R. D. King A. O. Chen C. Y. Corley E. G. Foster B. S. Roberts F. E. Yang C. Lieberman D. R. Reamer R. A. Tschaen D. M. Verhoeven T. R. Reider P. J. Lo Y. S. Rossano L. T. Brookes A. S. Meloni D. Moore J. R. Arnett J. F. J. Org. Chem. 1994;59:6391. doi: 10.1021/jo00100a048. - DOI
LinkOut - more resources
Full Text Sources

