Targeted protein oxidation using a chromophore-modified rapamycin analog
- PMID: 34777761
- PMCID: PMC8528027
- DOI: 10.1039/d1sc04464h
Targeted protein oxidation using a chromophore-modified rapamycin analog
Abstract
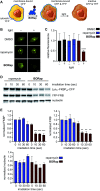
Chemically induced dimerization of FKBP and FRB using rapamycin and rapamycin analogs has been utilized in a variety of biological applications. Formation of the FKBP-rapamycin-FRB ternary complex is typically used to activate a biological process and this interaction has proven to be essentially irreversible. In many cases, it would be beneficial to also have temporal control over deactivating a biological process once it has been initiated. Thus, we developed the first reactive oxygen species-generating rapamycin analog toward this goal. The BODIPY-rapamycin analog BORap is capable of dimerizing FKBP and FRB to form a ternary complex, and upon irradiation with 530 nm light, generates singlet oxygen to oxidize and inactivate proteins of interest fused to FKBP/FRB.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




References
-
- Wang P. Gao Z. Yuan M. Zhu J. Wang F. Mechanically linked poly[2]rotaxanes constructed from the benzo-21-crown-7/secondary ammonium salt recognition motif. Polym. Chem. 2016;7(22):3664–3668. doi: 10.1039/C6PY00494F. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

