Role of rare-earth elements in enhancing bioelectrocatalysis for biosensing with NAD+-dependent glutamate dehydrogenase
- PMID: 34777762
- PMCID: PMC8528072
- DOI: 10.1039/d1sc00193k
Role of rare-earth elements in enhancing bioelectrocatalysis for biosensing with NAD+-dependent glutamate dehydrogenase
Abstract
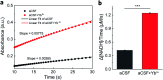
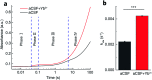
Dehydrogenases (DHs) are widely explored bioelectrocatalysts in the development of enzymatic bioelectronics like biosensors and biofuel cells. However, the relatively low intrinsic reaction rates of DHs which mostly depend on diffusional coenzymes (e.g., NAD+) have limited their bioelectrocatalytic performance in applications such as biosensors with a high sensitivity. In this study, we find that rare-earth elements (REEs) can enhance the activity of NAD+-dependent glutamate dehydrogenase (GDH) toward highly sensitive electrochemical biosensing of glutamate in vivo. Electrochemical studies show that the sensitivity of the GDH-based glutamate biosensor is remarkably enhanced in the presence of REE cations (i.e., Yb3+, La3+ or Eu3+) in solution, of which Yb3+ yields the highest sensitivity increase (ca. 95%). With the potential effect of REE cations on NAD+ electrochemistry being ruled out, homogeneous kinetic assays by steady-state and stopped-flow spectroscopy reveal a two-fold enhancement in the intrinsic reaction rate of GDH by introducing Yb3+, mainly through accelerating the rate-determining NADH releasing step during the catalytic cycle. In-depth structural investigations using small angle X-ray scattering and infrared spectroscopy indicate that Yb3+ induces the backbone compaction of GDH and subtle β-sheet transitions in the active site, which may reduce the energetic barrier to NADH dissociation from the binding pocket as further suggested by molecular dynamics simulation. This study not only unmasks the mechanism of REE-promoted GDH kinetics but also paves a new way to highly sensitive biosensing of glutamate in vivo.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
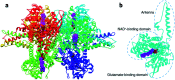



Similar articles
-
Noncovalent attachment of NAD+ cofactor onto carbon nanotubes for preparation of integrated dehydrogenase-based electrochemical biosensors.Langmuir. 2010 Apr 20;26(8):6028-32. doi: 10.1021/la903799n. Langmuir. 2010. PMID: 20121055
-
Ammonia utilization by a proposed bacterial pathogen in human periodontal disease, Capnocytophaga ochracea.Arch Oral Biol. 1983;28(4):327-38. doi: 10.1016/0003-9969(83)90075-4. Arch Oral Biol. 1983. PMID: 6576737
-
Structural and functional insights into the flexible β-hairpin of glycerol dehydrogenase.FEBS J. 2023 Sep;290(17):4342-4355. doi: 10.1111/febs.16813. Epub 2023 May 19. FEBS J. 2023. PMID: 37165682
-
Glutamate dehydrogenases: the why and how of coenzyme specificity.Neurochem Res. 2014;39(3):426-32. doi: 10.1007/s11064-013-1089-x. Epub 2013 Jun 13. Neurochem Res. 2014. PMID: 23761034 Review.
-
NAD(P)-dependent glucose dehydrogenase: Applications for biosensors, bioelectrodes, and biofuel cells.Bioelectrochemistry. 2020 Oct;135:107574. doi: 10.1016/j.bioelechem.2020.107574. Epub 2020 May 23. Bioelectrochemistry. 2020. PMID: 32498025 Review.
Cited by
-
Current applications and future perspectives on rare-earth-based materials in stomatology.iScience. 2025 Jul 26;28(9):113220. doi: 10.1016/j.isci.2025.113220. eCollection 2025 Sep 19. iScience. 2025. PMID: 40822902 Free PMC article. Review.
References
-
- Xia L. Van Nguyen K. Holade Y. Han H. Dooley K. Atanassov P. Banta S. Minteer S. D. ACS Energy Lett. 2017;2:1435–1438. doi: 10.1021/acsenergylett.7b00134. - DOI
-
- Ulyanova Y. Arugula M. A. Rasmussen M. Pinchon E. Lindstrom U. Singhal S. Minteer S. D. ACS Catal. 2014;4:4289–4294. doi: 10.1021/cs500802d. - DOI
LinkOut - more resources
Full Text Sources

