Cobalt-catalyzed multisubstituted allylation of the chelation-assisted C-H bond of (hetero)arenes with cyclopropenes
- PMID: 34777763
- PMCID: PMC8528013
- DOI: 10.1039/d1sc03476f
Cobalt-catalyzed multisubstituted allylation of the chelation-assisted C-H bond of (hetero)arenes with cyclopropenes
Abstract
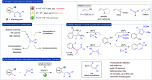
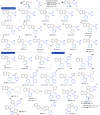
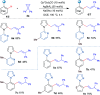
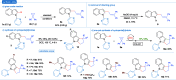
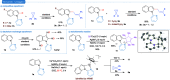
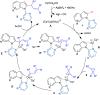
Cyclopropenes are highly strained three-membered carbocycles, which offer unique reactivity in organic synthesis. Herein, Cp*CoIII-catalyzed ring-opening isomerization of cyclopropenes to cobalt vinylcarbene has been utilized for the synthesis of multisubstituted allylarenes via directing group-assisted functionalization of C-H bonds of arenes and heteroarenes. Employing this methodology, various substituents can be introduced at all three carbons of the allyl moiety with high selectivity. The important highlights are excellent functional group tolerance, multisubstituted allylation, high selectivity, gram scale synthesis, removable directing group, and synthesis of cyclopenta[b]indoles. In addition, a potential cobaltocycle intermediate was identified and a plausible mechanism is also proposed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Sohn H. Y. Son K. H. Kwon C. S. Kwon G. S. Kang S. S. Phytomedicine. 2004;11:666–672. doi: 10.1016/j.phymed.2003.09.005. - DOI - PubMed
- Du J. He Z.-D. Jiang R.-W. Ye W.-C. Xu H.-X. But P. P.-H. Phytochemistry. 2003;62:1235–1238. doi: 10.1016/S0031-9422(02)00753-7. - DOI - PubMed
- Ni G. Zhang Q.-J. Zheng Z.-F. Chen R.-Y. Yu D.-Q. J. Nat. Prod. 2009;72:966–968. doi: 10.1021/np800789y. - DOI - PubMed
-
- Kiyotsuka Y. Acharya H. P. Katayama Y. Hyodo T. Kobayashi Y. Org. Lett. 2008;10:1719–1722. doi: 10.1021/ol800300x. - DOI - PubMed
- Spino C. Tremblay M.-C. Gobdout C. Org. Lett. 2004;6:2801–2804. doi: 10.1021/ol048936q. - DOI - PubMed
- Harrington-Frost N. Leuser H. Calaza M. I. Kneisel F. F. Knochel P. Org. Lett. 2003;5:2111–2114. doi: 10.1021/ol034525i. - DOI - PubMed
-
- Lee S. Y. Hartwig J. F. J. Am. Chem. Soc. 2016;138:15278–15284. doi: 10.1021/jacs.6b10220. - DOI - PMC - PubMed
- Bae S. Jang H.-L. Jung H. Joo J. M. J. Org. Chem. 2015;80:690–697. doi: 10.1021/jo5025317. - DOI - PubMed
- Fan S. Chen F. Zhang X. Angew. Chem., Int. Ed. 2011;50:5918–5923. doi: 10.1002/anie.201008174. - DOI - PubMed
- Achar T. K. Zhang X. Mondal R. Shanavas M. S. Maiti S. Maity S. Pal N. Paton R. S. Maiti D. Angew. Chem., Int. Ed. 2019;58:10353–10360. doi: 10.1002/anie.201904608. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

