Real-time imaging of cell-surface proteins with antibody-based fluorogenic probes
- PMID: 34777767
- PMCID: PMC8528012
- DOI: 10.1039/d1sc03065e
Real-time imaging of cell-surface proteins with antibody-based fluorogenic probes
Abstract
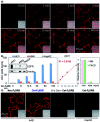
Cell-surface proteins, working as key agents in various diseases, are the targets for around 66% of approved human drugs. A general strategy to selectively detect these proteins in a real-time manner is expected to facilitate the development of new drugs and medical diagnoses. Although brilliant successes were attained using small-molecule probes, they could cover a narrow range of targets due to the lack of suitable ligands and some of them suffer from selectivity issues. We report herein an antibody-based fluorogenic probe prepared via a two-step chemical modification under physiological conditions, to fulfill the selective recognition and wash-free imaging of membrane proteins, establishing a modular strategy with broad implications for biochemical research and for therapeutics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Gao J. Hori Y. Nishiura M. Bordy M. Hasserodt J. Kikuchi K. Chem. Lett. 2020;49:232–235. doi: 10.1246/cl.190875. - DOI
LinkOut - more resources
Full Text Sources

