Depolymerization of supramolecular polymers by a covalent reaction; transforming an intercalator into a sequestrator
- PMID: 34777777
- PMCID: PMC8528007
- DOI: 10.1039/d1sc04545h
Depolymerization of supramolecular polymers by a covalent reaction; transforming an intercalator into a sequestrator
Abstract
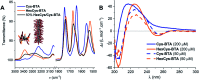
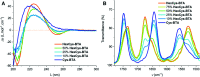
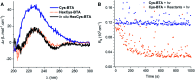
Controlling the reciprocity between chemical reactivity and supramolecular structure is a topic of great interest in the emergence of molecular complexity. In this work, we investigate the effect of a covalent reaction as a trigger to depolymerize a supramolecular assembly. We focus on the impact of an in situ thiol-ene reaction on the (co)polymerization of three derivatives of benzene-1,3,5-tricarboxamide (BTA) monomers functionalized with cysteine, hexylcysteine, and alkyl side chains: Cys-BTA, HexCys-BTA, and a-BTA. Long supramolecular polymers of Cys-BTA can be depolymerized into short dimeric aggregates of HexCys-BTA via the in situ thiol-ene reaction. Analysis of the system by time-resolved spectroscopy and light scattering unravels the fast dynamicity of the structures and the mechanism of depolymerization. Moreover, by intercalating the reactive Cys-BTA monomer into an unreactive inert polymer, the in situ thiol-ene reaction transforms the intercalator into a sequestrator and induces the depolymerization of the unreactive polymer. This work shows that the implementation of reactivity into supramolecular assemblies enables temporal control of depolymerization processes, which can bring us one step closer to understanding the interplay between non-covalent and covalent chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

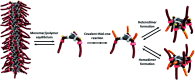

Similar articles
-
Tuning the Length of Cooperative Supramolecular Polymers under Thermodynamic Control.J Am Chem Soc. 2019 Nov 13;141(45):18278-18285. doi: 10.1021/jacs.9b09443. Epub 2019 Nov 4. J Am Chem Soc. 2019. PMID: 31638390 Free PMC article.
-
Photodynamic Control of the Chain Length in Supramolecular Polymers: Switching an Intercalator into a Chain Capper.J Am Chem Soc. 2020 Apr 1;142(13):6295-6303. doi: 10.1021/jacs.0c00858. Epub 2020 Mar 20. J Am Chem Soc. 2020. PMID: 32167302 Free PMC article.
-
In situ Synthesis of Supramolecular Polymers: Finding the Right Conditions when Combining Covalent and Non-Covalent Synthesis.Angew Chem Int Ed Engl. 2022 Aug 22;61(34):e202206729. doi: 10.1002/anie.202206729. Epub 2022 Jul 14. Angew Chem Int Ed Engl. 2022. PMID: 35763321 Free PMC article.
-
Synergistic Assembly of Covalent and Supramolecular Polymers.Macromol Rapid Commun. 2016 Jun;37(11):920-3. doi: 10.1002/marc.201600153. Epub 2016 Apr 14. Macromol Rapid Commun. 2016. PMID: 27076255 Review.
-
Secondary Structure in Nonpeptidic Supramolecular Block Copolymers.Acc Chem Res. 2021 May 18;54(10):2397-2408. doi: 10.1021/acs.accounts.1c00028. Epub 2021 Apr 29. Acc Chem Res. 2021. PMID: 33914498 Review.
Cited by
-
Hierarchical self-assembly of aromatic peptide conjugates into supramolecular polymers: it takes two to tango.Chem Sci. 2021 Dec 10;13(4):909-933. doi: 10.1039/d1sc05589e. eCollection 2022 Jan 26. Chem Sci. 2021. PMID: 35211257 Free PMC article. Review.
-
Enhanced Stability and Properties of Benzene-1,3,5-Tricarboxamide Supramolecular Copolymers through Engineered Coupled Equilibria.Angew Chem Int Ed Engl. 2025 Mar 17;64(12):e202421991. doi: 10.1002/anie.202421991. Epub 2024 Dec 8. Angew Chem Int Ed Engl. 2025. PMID: 39569591 Free PMC article.
-
Site selectivity steps in.Nat Chem. 2023 Jul;15(7):894-895. doi: 10.1038/s41557-023-01237-7. Nat Chem. 2023. PMID: 37402790 No abstract available.
-
Elucidating the Boundary of Intercalation vs Sequestration in Supramolecular Polymers by Retrosynthetic Design Toward the Construction of Complex Supramolecular Systems.Angew Chem Int Ed Engl. 2025 May;64(19):e202501693. doi: 10.1002/anie.202501693. Epub 2025 Mar 10. Angew Chem Int Ed Engl. 2025. PMID: 39985367 Free PMC article.
-
Spatiotemporal dynamics of supramolecular polymers by in situ quantitative catalyst-free hydroamination.Chem Sci. 2022 Mar 23;13(15):4413-4423. doi: 10.1039/d2sc00035k. eCollection 2022 Apr 13. Chem Sci. 2022. PMID: 35509456 Free PMC article.
References
-
- Hartlieb M. Mansfield E. D. H. Perrier S. Polym. Chem. 2020;11:1083–1110. doi: 10.1039/C9PY01342C. - DOI
-
- Walsh C. T., Posttranslational Modification of Proteins, Roberts & Company Publishers, Greenwood Village, 2006
LinkOut - more resources
Full Text Sources

