Evidence supporting a role for circulating macrophages in the regression of vascular remodeling following sub-chronic exposure to hemoglobin plus hypoxia
- PMID: 34777787
- PMCID: PMC8573496
- DOI: 10.1177/20458940211056806
Evidence supporting a role for circulating macrophages in the regression of vascular remodeling following sub-chronic exposure to hemoglobin plus hypoxia
Erratum in
-
Corrigendum for Evidence supporting a role for circulating macrophages in the regression of vascular remodeling following sub-chronic exposure to hemoglobin plus hypoxia.Pulm Circ. 2021 Dec 13;11(4):20458940211068399. doi: 10.1177/20458940211068399. eCollection 2021 Oct-Dec. Pulm Circ. 2021. PMID: 34925764 Free PMC article.
Abstract
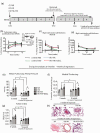
Macrophages are a heterogeneous population with both pro- and anti-inflammatory functions play an essential role in maintaining tissue homeostasis, promoting inflammation under pathological conditions, and tissue repair after injury. In pulmonary hypertension, the M1 phenotype is more pro-inflammatory compared to the M2 phenotype, which is involved in tissue repair. The role of macrophages in the initiation and progression of pulmonary hypertension is well studied. However, their role in the regression of established pulmonary hypertension is not well known. Rats chronically exposed to hemoglobin (Hb) plus hypoxia (HX) share similarities to humans with pulmonary hypertension associated with hemolytic disease, including the presence of a unique macrophage phenotype surrounding distal vessels that are associated with vascular remodeling. These lung macrophages are characterized by high iron content, HO-1, ET-1, and IL-6, and are recruited from the circulation. Depletion of macrophages in this model prevents the development of pulmonary hypertension and vascular remodeling. In this study, we specifically investigate the regression of pulmonary hypertension over a four-week duration after rats were removed from Hb + HX exposure with and without gadolinium chloride administration. Withdrawal of Hb + HX reversed systolic pressures and right ventricular function after Hb + Hx exposure in four weeks. Our data show that depleting circulating monocytes/macrophages during reversal prevents complete recovery of right ventricular systolic pressure and vascular remodeling in this rat model of pulmonary hypertension at four weeks post exposure. The data presented offer a novel insight into the role of macrophages in the processes of pulmonary hypertension regression in a rodent model of Hb + Hx-driven disease.
Keywords: heart; hemoglobinopathies; lung; pulmonary vascular disease; sickle cell disease.
© The Author(s) 2021.
Figures



References
-
- Tuder RM, Stacher E, Robinson J, et al. Pathology of pulmonary hypertension. Clin Chest Med 2013; 34: 639–650. - PubMed
-
- Rother RP, Bell L, Hillmen P, et al. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 2005; 293: 1653–1662. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

