GH47 and Other Glycoside Hydrolases Catalyze Glycosidic Bond Cleavage with the Assistance of Substrate Super-arming at the Transition State
- PMID: 34777906
- PMCID: PMC8579916
- DOI: 10.1021/acscatal.1c02750
GH47 and Other Glycoside Hydrolases Catalyze Glycosidic Bond Cleavage with the Assistance of Substrate Super-arming at the Transition State
Abstract
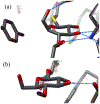
Super-armed glycosyl donors, whose substituents are predominantly held in pseudoaxial positions, exhibit strongly increased reactivity in glycosylation through significant stabilization of oxocarbenium-like transition states. Examination of X-ray crystal structures reveals that the GH47 family of glycoside hydrolases have evolved so as to distort their substrates away from the ground state conformation in such a manner as to present multiple C-O bonds in pseudoaxial positions and so benefit from conformational super-arming of their substrates, thereby enhancing catalysis. Through analysis of literature mutagenic studies, we show that a suitably placed aromatic residue in GHs 6 and 47 sterically enforces super-armed conformations on their substrates. GH families 45, 81, and 134 on the other hand impose conformational super-arming on their substrates, by maintaining the more active ring conformation through hydrogen bonding rather than steric interactions. The recognition of substrate super-arming by select GH families provides a further parallel with synthetic carbohydrate chemistry and nature and opens further avenues for the design of improved glycosidase inhibitors.
Keywords: CH-π interactions; Glycoside hydrolases; conformational analysis; electrostatic transition state stabilization; half-chair; super-arming; twist-boat; α-mannosidases.
Figures


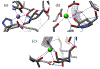


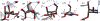

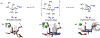
References
-
- Zechel DL; Withers SG, Glycosidase Mechanisms: Anatomy of a Finely Tuned Catalyst. Acc. Chem. Res 2000, 33, 11–18. - PubMed
-
- Davies GJ; Planas A; Rovira C, Conformational Analyses of the Reaction Coordinate of Glycosidases. Acc. Chem. Res 2012, 45, 308–316. - PubMed
-
- Colombo C; Bennet AJ, Probing Transition State Analogy in Glycoside Hydrolase Catalysis. Adv. Phys. Org. Chem 2017, 51, 99–127.
-
- Wu L; Armstrong Z; Schröder SP; de Boer C; Artola M; Aerts JMFG; Overkleeft HS; Davies GJ, An Overview of Activity-Based Probes for Glycosidases. Curr. Opin. Chem. Biol 2019, 53, 25–36. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
