Processive Enzymes Kept on a Leash: How Cellulase Activity in Multienzyme Complexes Directs Nanoscale Deconstruction of Cellulose
- PMID: 34777910
- PMCID: PMC8576811
- DOI: 10.1021/acscatal.1c03465
Processive Enzymes Kept on a Leash: How Cellulase Activity in Multienzyme Complexes Directs Nanoscale Deconstruction of Cellulose
Abstract
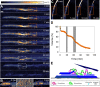
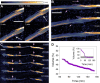
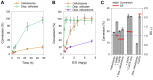
Biological deconstruction of polymer materials gains efficiency from the spatiotemporally coordinated action of enzymes with synergetic function in polymer chain depolymerization. To perpetuate enzyme synergy on a solid substrate undergoing deconstruction, the overall attack must alternate between focusing the individual enzymes locally and dissipating them again to other surface sites. Natural cellulases working as multienzyme complexes assembled on a scaffold protein (the cellulosome) maximize the effect of local concentration yet restrain the dispersion of individual enzymes. Here, with evidence from real-time atomic force microscopy to track nanoscale deconstruction of single cellulose fibers, we show that the cellulosome forces the fiber degradation into the transversal direction, to produce smaller fragments from multiple local attacks ("cuts"). Noncomplexed enzymes, as in fungal cellulases or obtained by dissociating the cellulosome, release the confining force so that fiber degradation proceeds laterally, observed as directed ablation of surface fibrils and leading to whole fiber "thinning". Processive cellulases that are enabled to freely disperse evoke the lateral degradation and determine its efficiency. Our results suggest that among natural cellulases, the dispersed enzymes are more generally and globally effective in depolymerization, while the cellulosome represents a specialized, fiber-fragmenting machinery.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Ragauskas A. J.; Williams C. K.; Davison B. H.; Britovsek G.; Cairney J.; Eckert C. A.; Frederick W. J. Jr; Hallett J. P.; Leak D. J.; Liotta C. L.; Mielenz J. R.; Murphy R.; Templer R.; Tschaplinski T. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. 10.1126/science.1114736. - DOI - PubMed
-
- Liao Y.; Koelewijn S. F.; van den Bossche G.; van Aelst J.; van den Bosch S.; Renders T.; Navare K.; Nicolaï T.; van Aelst K.; Maesen M.; Matsushima H.; Thevelein J. M.; van Acker K.; Lagrain B.; Verboekend D.; Sels B. F. A sustainable wood biorefinery for low-carbon footprint chemicals production. Science 2020, 367, 1385–1390. 10.1126/science.aau1567. - DOI - PubMed
-
- Alonso D. M.; Hakim S. H.; Zhou S.; Won W.; Hosseinaei O.; Tao J.; Garcia-Negron V.; Motagamwala A. H.; Mellmer M. A.; Huang K.; Houtman C. J.; Labbé N.; Harper D. P.; Maravelias C. T.; Runge T.; Dumesic J. A. Increasing the revenue from lignocellulosic biomass: maximizing feedstock utilization. Sci. Adv. 2017, 3, e1603301 10.1126/sciadv.1603301. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
