Does the Configuration at the Metal Matter in Noyori-Ikariya Type Asymmetric Transfer Hydrogenation Catalysts?
- PMID: 34777911
- PMCID: PMC8576814
- DOI: 10.1021/acscatal.1c03636
Does the Configuration at the Metal Matter in Noyori-Ikariya Type Asymmetric Transfer Hydrogenation Catalysts?
Abstract
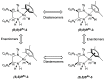
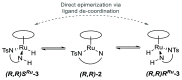
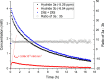
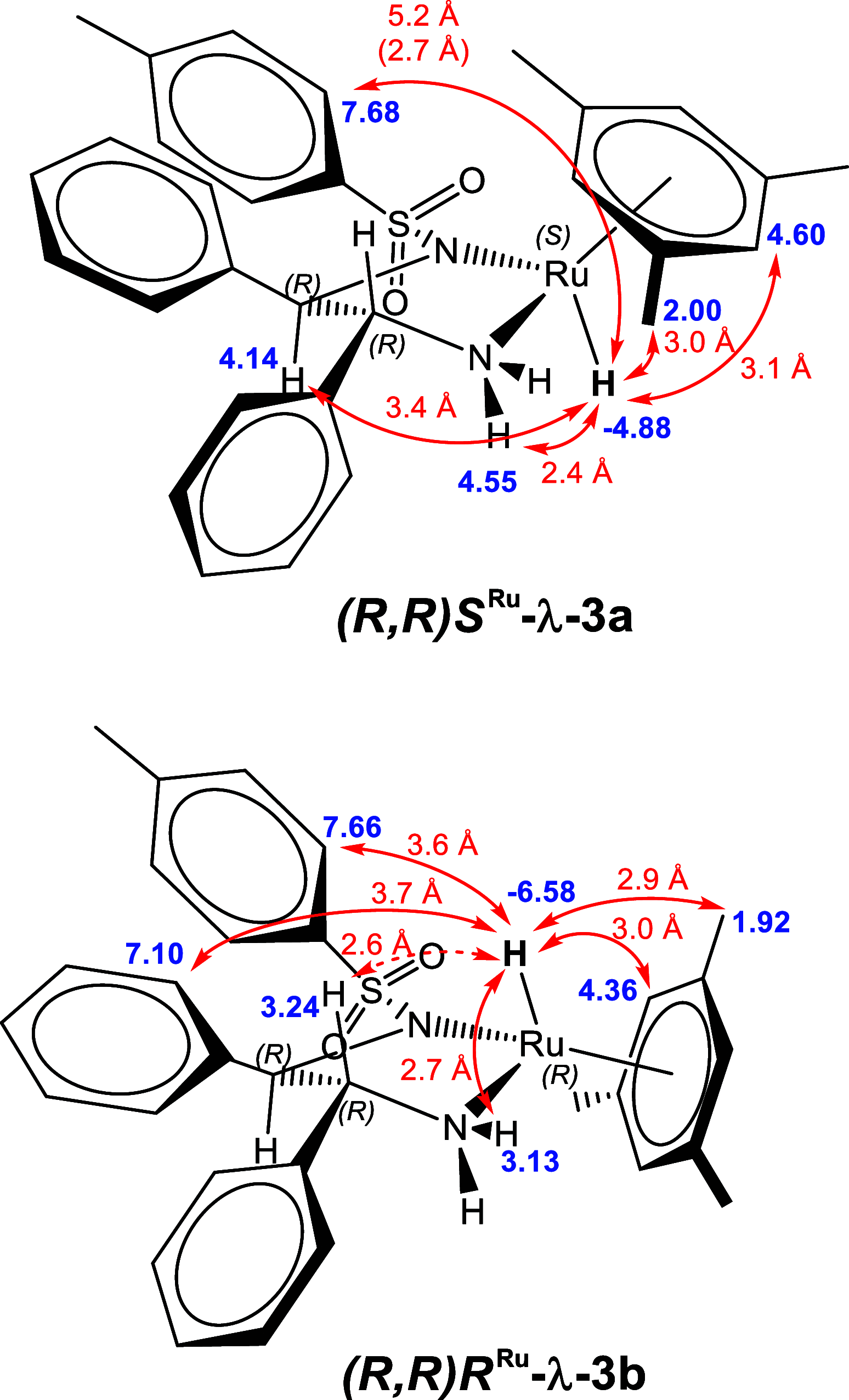
Noyori-Ikariya type [(arene)RuCl(TsDPEN)] (TsDPEN, sulfonated diphenyl ethylenediamine) complexes are widely used C=O and C=N reduction catalysts that produce chiral alcohols and amines via a key ruthenium-hydride intermediate that determines the stereochemistry of the product. Whereas many details about the interactions of the pro-chiral substrate with the hydride complex and the nature of the hydrogen transfer from the latter to the former have been investigated over the past 25 years, the role of the stereochemical configuration at the stereogenic ruthenium center in the catalysis has not been elucidated so far. Using operando FlowNMR spectroscopy and nuclear Overhauser effect spectroscopy, we show the existence of two diastereomeric hydride complexes under reaction conditions, assign their absolute configurations in solution, and monitor their interconversion during transfer hydrogenation catalysis. Configurational analysis and multifunctional density functional theory (DFT) calculations show the λ-(R,R)S Ru configured [(mesitylene)RuH(TsDPEN)] complex to be both thermodynamically and kinetically favored over its λ-(R,R)R Ru isomer with the opposite configuration at the metal. Computational analysis of both diastereomeric catalytic manifolds show the major λ-(R,R)S Ru configured [(mesitylene)RuH(TsDPEN)] complex to dominate asymmetric ketone reduction catalysis with the minor λ-(R,R)R Ru [(mesitylene)RuH(TsDPEN)] stereoisomer being both less active and less enantioselective. These findings also hold true for a tethered catalyst derivative with a propyl linker between the arene and TsDPEN ligands and thus show enantioselective transfer hydrogenation catalysis with Noyori-Ikariya complexes to proceed via a lock-and-key mechanism.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): A.C. and I.C. are employees of Bruker UK Ltd., manufacturer and supplier of NMR hard- and software solutions that have been used in this research. The other authors declare no competing financial interest.
Figures
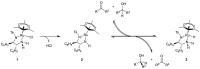





Similar articles
-
Insight into catalyst speciation and hydrogen co-evolution during enantioselective formic acid-driven transfer hydrogenation with bifunctional ruthenium complexes from multi-technique operando reaction monitoring.Faraday Discuss. 2019 Dec 2;220(0):45-57. doi: 10.1039/c9fd00060g. Faraday Discuss. 2019. PMID: 31524899
-
Density functional theory investigation of Ru(II) and Os(II) asymmetric transfer hydrogenation catalysts.Faraday Discuss. 2022 May 18;234(0):264-283. doi: 10.1039/d1fd00075f. Faraday Discuss. 2022. PMID: 35156974
-
A multilateral mechanistic study into asymmetric transfer hydrogenation in water.Chemistry. 2008;14(25):7699-715. doi: 10.1002/chem.200800559. Chemistry. 2008. PMID: 18604853
-
Experimental and theoretical perspectives of the Noyori-Ikariya asymmetric transfer hydrogenation of imines.Molecules. 2014 May 28;19(6):6987-7007. doi: 10.3390/molecules19066987. Molecules. 2014. PMID: 24879612 Free PMC article. Review.
-
Opportunities offered by chiral η⁶-arene/N-arylsulfonyl-diamine-RuII catalysts in the asymmetric transfer hydrogenation of ketones and imines.Molecules. 2011 Jun 28;16(7):5460-95. doi: 10.3390/molecules16075460. Molecules. 2011. PMID: 21712760 Free PMC article. Review.
Cited by
-
Catalytic Asymmetric Transfer Hydrogenation of β,γ-Unsaturated α-Diketones.J Am Chem Soc. 2024 Dec 11;146(49):33543-33560. doi: 10.1021/jacs.4c11070. Epub 2024 Nov 27. J Am Chem Soc. 2024. PMID: 39604061 Free PMC article.
-
Catalytic Stereoconvergent Synthesis of Homochiral β-CF3, β-SCF3, and β-OCF3 Benzylic Alcohols.ACS Org Inorg Au. 2022 Oct 5;2(5):396-404. doi: 10.1021/acsorginorgau.2c00019. Epub 2022 Jun 8. ACS Org Inorg Au. 2022. PMID: 36217345 Free PMC article.
-
Illuminating the multiple Lewis acidity of triaryl-boranes via atropisomeric dative adducts.Chem Sci. 2024 Aug 26;15(38):15679-89. doi: 10.1039/d4sc00925h. Online ahead of print. Chem Sci. 2024. PMID: 39257854 Free PMC article.
-
Effect of Ligand Substituents on Spectroscopic and Catalytic Properties of Water-Compatible Cp*Ir-(pyridinylmethyl)sulfonamide-Based Transfer Hydrogenation Catalysts.Inorg Chem. 2024 Feb 26;63(8):3815-3823. doi: 10.1021/acs.inorgchem.3c04040. Epub 2024 Feb 11. Inorg Chem. 2024. PMID: 38343274 Free PMC article.
-
Metal Stereogenicity in Asymmetric Transition Metal Catalysis.Chem Rev. 2023 Apr 26;123(8):4764-4794. doi: 10.1021/acs.chemrev.2c00724. Epub 2023 Mar 29. Chem Rev. 2023. PMID: 36988612 Free PMC article. Review.
References
-
- Eisenstein O.; Crabtree R. H. Outer sphere hydrogenation catalysis. New J. Chem. 2013, 37, 21–27. 10.1039/C2NJ40659D. - DOI
-
- Gridnev I. D.; Dub P. A.. Enantioselection in Asymmetric Catalysis, 1st ed.; CRC Press, 2016; p 246.
-
- Haack K.-J.; Hashiguchi S.; Fujii A.; Ikariya T.; Noyori R. The Catalyst Precursor, Catalyst and Intermediate in the Ru(II)-Promoted Asymmetric Hydrogen Transfer between Alcohols and Ketones. Angew. Chem., Int. Ed. Engl. 1997, 36, 285–288. 10.1002/anie.199702851. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
