Economic Evaluation of Sacituzumab Govitecan for the Treatment of Metastatic Triple-Negative Breast Cancer in China and the US
- PMID: 34778047
- PMCID: PMC8581633
- DOI: 10.3389/fonc.2021.734594
Economic Evaluation of Sacituzumab Govitecan for the Treatment of Metastatic Triple-Negative Breast Cancer in China and the US
Abstract
Background: The effectiveness of Sacituzumab Govitecan (SG) for metastatic triple-negative breast cancer (mTNBC) has been demonstrated. We aimed to evaluate its cost-effectiveness on mTNBC from the Chinese and United States (US) perspective.
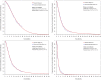
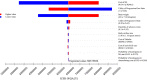
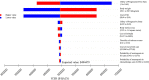
Methods: A partitioned survival model was developed to compare the cost and effectiveness of SG versus single-agent chemotherapy based on clinical data from the ASCENT phase 3 randomized trial. Cost and utility data were obtained from the literature. The incremental cost-effectiveness ratio (ICER) was measured, and one-way and probabilistic sensitivity analyses (PSA) were performed to observe model stability. A Markov model was constructed to validate the results.
Results: In China, SG yielded an additional 0.35 quality-adjusted life-year (QALY) at an additional cost of Chinese Renminbi ¥2257842. The ICER was ¥6375856 ($924037)/QALY. In the US, SG yielded the same additional QALY at an extra cost of $175393 and the ICER was $494479/QALY. Similar results were obtained from the Markov model. One-way sensitivity analyses showed that SG price had the greatest impact on the ICER. PSA showed the probability of SG to be cost-effective when compared with chemotherapy was zero at the current willing-to-pay threshold of ¥217341/QALY and $150000/QALY in China and the US, respectively. The probability of cost-effectiveness of SG would approximate 50% if its price was reduced to ¥10.44/mg in China and $3.65/mg in the US.
Conclusion: SG is unlikely to be a cost-effective treatment of mTNBC at the current price both in China and the US.
Keywords: China; Sacituzumab Govitecan; US; breast cancer; economic evaluation.
Copyright © 2021 Chen, Han, Liu and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Garrido-Castro AC, Lin NU, Polyak K. Insights Into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov (2019) 9(2):176–98. doi: 10.1158/2159-8290.CD-18-1177 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

