Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications
- PMID: 34778089
- PMCID: PMC8581618
- DOI: 10.3389/fonc.2021.770561
Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications
Abstract
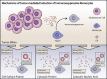
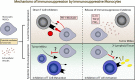
Glioblastoma (GBM) is the most common primary brain tumor in adults an carries and carries a terrible prognosis. The current regiment of surgical resection, radiation, and chemotherapy has remained largely unchanged in recent years as new therapeutic approaches have struggled to demonstrate benefit. One of the most challenging hurdles to overcome in developing novel treatments is the profound immune suppression found in many GBM patients. This limits the utility of all manner of immunotherapeutic agents, which have revolutionized the treatment of a number of cancers in recent years, but have failed to show similar benefit in GBM therapy. Understanding the mechanisms of tumor-mediated immune suppression in GBM is critical to the development of effective novel therapies, and reversal of this effect may prove key to effective immunotherapy for GBM. In this review, we discuss the current understanding of tumor-mediated immune suppression in GBM in both the local tumor microenvironment and systemically. We also discuss the effects of current GBM therapy on the immune system. We specifically explore some of the downstream effectors of tumor-driven immune suppression, particularly myeloid-derived suppressor cells (MDSCs) and other immunosuppressive monocytes, and the manner by which GBM induces their formation, with particular attention to the role of GBM-derived extracellular vesicles (EVs). Lastly, we briefly review the current state of immunotherapy for GBM and discuss additional hurdles to overcome identification and implementation of effective therapeutic strategies.
Keywords: extracellular vesicles; glioblastoma; immunosuppression; immunotherapy; myeloid - derived suppressor cell.
Copyright © 2021 Himes, Geiger, Ayasoufi, Bhargav, Brown and Parney.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. . Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA (2017) 318:2306–16. doi: 10.1001/jama.2017.18718 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

