Promelaxin Microenemas Are Non-inferior to Oral Polyethylene Glycol for the Treatment of Functional Constipation in Young Children: A Randomized Clinical Trial
- PMID: 34778144
- PMCID: PMC8586088
- DOI: 10.3389/fped.2021.753938
Promelaxin Microenemas Are Non-inferior to Oral Polyethylene Glycol for the Treatment of Functional Constipation in Young Children: A Randomized Clinical Trial
Abstract
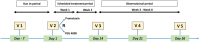
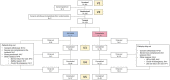
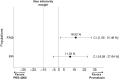
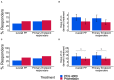
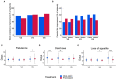
Background: Polyethylene glycol (PEG) is recommended as first-line treatment of pediatric functional constipation. However, the oral route of administration is often poorly feasible in children mostly due to poor palatability. Promelaxin microenemas exert a topical evacuative action and may offer a valuable option in pediatric FC. Aim: To assess whether Promelaxin microenemas would be non-inferior to PEG 4000 in young children with FC. Methods: This is a randomized, open-label, multi-centric, non-inferiority trial enrolling infants and young children aged 6-48 months, with FC according to Rome III criteria. After 1 week of run in, children were randomized to 2 weeks of Promelaxin or PEG, followed by a 6-week on-demand treatment period. Primary endpoint was response rate to randomized treatment, with "response" defined as at least 3 evacuations per week and an average increase of at least one evacuation per week as compared to baseline. Safety, stool consistency and the analysis of fecal microbiota were secondary endpoints. Results: Out of the 158 patients who entered the trial, 153 patients were treated (77 and 76, PEG and Promelaxin arm, respectively). In the primary analysis, the 95% confidence interval (CI) for the treatment's effect lay entirely above the non-inferiority margin in both Full Set (FAS) and Per Protocol (PP) analyses, providing evidence of the non-inferiority of Promelaxin vs. PEG 4000 [response rate difference: 16.5% (CI 1.55-31.49%) and 11.03% (CI -5.58 to 27.64%), FAS and PP analyses, respectively]. Mean compliance to the randomized treatment was >80% in both arms. Secondary endpoints did not significantly differ between the two arms, except for the average number of total days of on-demand treatment that was significantly lower in the Promelaxin arm [14.6 (12.7) vs. 9.8 (9.1), mean (SD); primary endpoint responders in PEG and Promelaxin arm, respectively; p = 0.027]. Microbiota evenness significantly increased in the PEG 4000 arm at V4 as compared to the Promelaxin arm (p < 0.05). In addition, at V5, patients treated with PEG showed a significantly decreased microbiota density as compared to patients treated with Promelaxin (p = 0.036). Conclusions: Promelaxin microenemas are non-inferior to oral PEG in children with FC. Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT02751411.
Keywords: Promelaxin microenemas; functional constipation; medical devices based on substances; polyethylene glycol; young children.
Copyright © 2021 Strisciuglio, Coppola, Russo, Tolone, Marseglia, Verrotti, Caimmi, Caloisi, D'Argenio, Sacchetti and Staiano.
Conflict of interest statement
AS is clinical investigator for Janssen Biologics B.V. and consultant for Angelini; she was clinical investigator for Aboca and PAREXEL International Srl; she was consultant for Aboca, for D.M.G. Italy and Nestlé, she was data safety monitoring board member for Sucampo AG and speaker for Aboca, Angelini, D.M.G. Italy and Valeas. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

