Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 Among Hospitalized Adults: Surveillance Protocol
- PMID: 34778173
- PMCID: PMC8585926
- DOI: 10.3389/fpubh.2021.739076
Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 Among Hospitalized Adults: Surveillance Protocol
Abstract
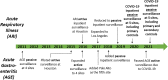
Introduction: Early in the COVID-19 pandemic, the Centers for Disease Control and Prevention (CDC) rapidly initiated COVID-19 surveillance by leveraging existing hospital networks to assess disease burden among hospitalized inpatients and inform prevention efforts. Materials and Methods: The Surveillance Platform for Enteric and Respiratory Infectious Organisms at Veterans Affairs Medical Centers (SUPERNOVA) is a network of five United States Veterans Affairs Medical Centers which serves nearly 400,000 Veterans annually and conducts laboratory-based passive and active monitoring for pathogens associated with acute gastroenteritis and acute respiratory illness among hospitalized Veterans. This paper presents surveillance methods for adapting the SUPERNOVA surveillance platform to prospectively evaluate COVID-19 epidemiology during a public health emergency, including detecting, characterizing, and monitoring patients with and without COVID-19 beginning in March 2020. To allow for case-control analyses, patients with COVID-19 and patients with non-COVID-19 acute respiratory illness were included. Results: SUPERNOVA included 1,235 participants with COVID-19 and 707 participants with other acute respiratory illnesses hospitalized during February through December 2020. Most participants were male (93.1%), with a median age of 70 years, and 45.8% non-Hispanic Black and 32.6% non-Hispanic White. Among those with COVID-19, 28.2% were transferred to an intensive care unit, 9.4% received invasive mechanical ventilation, and 13.9% died. Compared with controls, after adjusting for age, sex, and race/ethnicity, COVID-19 case-patients had significantly higher risk of mortality, respiratory failure, and invasive mechanical ventilation, and longer hospital stays. Discussion: Strengths of the SUPERNOVA platform for COVID-19 surveillance include the ability to collect and integrate multiple types of data, including clinical and illness outcome information, and SARS-CoV-2 laboratory test results from respiratory and serum specimens. Analysis of data from this platform also enables formal comparisons of participants with and without COVID-19. Surveillance data collected during a public health emergency from this key U.S. population of Veterans will be useful for epidemiologic investigations of COVID-19 spectrum of disease, underlying medical conditions, virus variants, and vaccine effectiveness, according to public health priorities and needs.
Keywords: COVID-19; Veterans health; epidemiology; public health; research methods; surveillance.
Copyright © 2021 Meites, Bajema, Kambhampati, Prill, Marconi, Brown, Rodriguez-Barradas, Beenhouwer, Holodniy, Lucero-Obusan, Cardemil, Cates, Surie and The SUPERNOVA COVID-19 Surveillance Group.
Conflict of interest statement
VM has received investigator-initiated research grants (to the institution) and consultation fees from Eli Lilly, Bayer, Gilead Sciences, and ViiV. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Veterans Health Administration. U.S. Department of Veterans Affairs. Available online at: https://www.va.gov/health (accessed March 30, 2021).
-
- Bagalman E. The Number of Veterans That Use VA Health Care Services: A Fact Sheet. Congressional Research Service, Contract No.: R43579; (2014).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

