Preclinical Application of Conditional Reprogramming Culture System for Laryngeal and Hypopharyngeal Carcinoma
- PMID: 34778255
- PMCID: PMC8585768
- DOI: 10.3389/fcell.2021.744969
Preclinical Application of Conditional Reprogramming Culture System for Laryngeal and Hypopharyngeal Carcinoma
Abstract
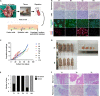
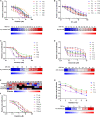
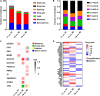
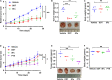
Management of laryngeal and hypopharyngeal squamous cell carcinoma (LHSCC) remains highly challenging due to highly variable therapeutic responses. By establishing an in vitro model for LHSCC based on conditional reprogramming (CR), a cell-culture technique, we aim to investigate its potential value on personalized cancer therapies. Herein, a panel of 28 human LHSCC CR cells were established from 50 tumor tissues using the CR method. They retained tumorigenic potential upon xenotransplantation and recapitulated molecular characteristics of LHSCC. Differential responses to anticancer drugs and radiotherapy were detected in vitro. CR cells could be transformed to xenograft and organoid, and they shared comparable drug responses. The clinical drug responses were consistent with in vitro drug responses. Collectively, the patient-derived CR cell model could promisingly be utilized in clinical decision-making and assisted in the selection of personalized therapies for LHSCC.
Keywords: conditional reprogramming; drug sensitivity; head and neck squamous cell carcinoma; in vitro model; personalized treatment.
Copyright © 2021 Dong, Wang, Ji, Zheng, Wang, Liu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
CT-based radiomics features in the prediction of thyroid cartilage invasion from laryngeal and hypopharyngeal squamous cell carcinoma.Cancer Imaging. 2020 Nov 11;20(1):81. doi: 10.1186/s40644-020-00359-2. Cancer Imaging. 2020. PMID: 33176885 Free PMC article.
-
Elevated expression of histone demethylase PHF8 associates with adverse prognosis in patients of laryngeal and hypopharyngeal squamous cell carcinoma.Epigenomics. 2015;7(2):143-53. doi: 10.2217/epi.14.82. Epub 2014 Dec 15. Epigenomics. 2015. PMID: 25496457
-
Prognostic value of lymph node ratio in laryngeal and hypopharyngeal squamous cell carcinoma: a systematic review and meta-analysis.J Otolaryngol Head Neck Surg. 2020 May 29;49(1):31. doi: 10.1186/s40463-020-00421-w. J Otolaryngol Head Neck Surg. 2020. PMID: 32471483 Free PMC article.
-
EGR1 regulates radiation-induced apoptosis in head and neck squamous cell carcinoma.Oncol Rep. 2015 Apr;33(4):1717-22. doi: 10.3892/or.2015.3747. Epub 2015 Jan 22. Oncol Rep. 2015. PMID: 25710185
-
[Organ preservation in advanced laryngeal/hypopharyngeal carcinoma: lessons from the DeLOS-II trial].HNO. 2020 Sep;68(9):648-656. doi: 10.1007/s00106-020-00890-5. HNO. 2020. PMID: 32468135 Review. German.
Cited by
-
Modeling respiratory tract diseases for clinical translation employing conditionally reprogrammed cells.Cell Insight. 2024 Sep 18;3(6):100201. doi: 10.1016/j.cellin.2024.100201. eCollection 2024 Dec. Cell Insight. 2024. PMID: 39391007 Free PMC article. Review.
-
Characterization of a Diverse Set of Conditionally Reprogrammed Head and Neck Cancer Cell Cultures.Laryngoscope. 2024 Jun;134(6):2748-2756. doi: 10.1002/lary.31236. Epub 2024 Jan 30. Laryngoscope. 2024. PMID: 38288866 Free PMC article.
-
Establishment of a diverse head and neck squamous cancer cell bank using conditional reprogramming culture methods.J Med Virol. 2023 Feb;95(2):e28388. doi: 10.1002/jmv.28388. J Med Virol. 2023. PMID: 36477880 Free PMC article.
References
-
- Dohmen A. J., Swartz J. E., Van Den Brekel M. W., Willems S. M., Spijker R., Neefjes J., et al. (2015). Feasibility of primary tumor culture models and preclinical prediction assays for head and neck cancer: a narrative review. Cancers (Basel) 7 1716–1742. 10.3390/cancers7030858 - DOI - PMC - PubMed
-
- Driehuis E., Kolders S., Spelier S., Lõhmussaar K., Willems S. M., Devriese L. A., et al. (2019). Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. 9 852–871. 10.1158/2159-8290.Cd-18-1522 - DOI - PubMed
LinkOut - more resources
Full Text Sources

