Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study
- PMID: 34778846
- PMCID: PMC8577228
- DOI: 10.1016/S2665-9913(21)00333-7
Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study
Abstract
Background: COVID-19 vaccines have robust immunogenicity in the general population. However, data for individuals with immune-mediated inflammatory diseases who are taking immunosuppressants remains scarce. Our previously published cohort study showed that methotrexate, but not targeted biologics, impaired functional humoral immunity to a single dose of COVID-19 vaccine BNT162b2 (Pfizer-BioNTech), whereas cellular responses were similar. Here, we aimed to assess immune responses following the second dose.
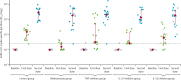
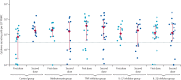
Methods: In this longitudinal cohort study, we recruited individuals with psoriasis who were receiving methotrexate or targeted biological monotherapy (ie, tumour necrosis factor [TNF] inhibitors, interleukin [IL]-17 inhibitors, or IL-23 inhibitors) from a specialist psoriasis centre serving London and South-East England. The healthy control cohort were volunteers without psoriasis, not receiving immunosuppression. Immunogenicity was evaluated immediately before, on day 28 after the first BNT162b2 vaccination and on day 14 after the second dose (administered according to an extended interval regimen). Here, we report immune responses following the second dose. The primary outcomes were humoral immunity to the SARS-CoV-2 spike glycoprotein, defined as titres of total spike-specific IgG and of neutralising antibody to wild-type, alpha (B.1.1.7), and delta (B.1.617.2) SARS-CoV-2 variants, and cellular immunity defined as spike-specific T-cell responses (including numbers of cells producing interferon-γ, IL-2, IL-21).
Findings: Between Jan 14 and April 4, 2021, 121 individuals were recruited, and data were available for 82 participants after the second vaccination. The study population included patients with psoriasis receiving methotrexate (n=14), TNF inhibitors (n=19), IL-17 inhibitors (n=14), IL-23 inhibitors (n=20), and 15 healthy controls, who had received both vaccine doses. The median age of the study population was 44 years (IQR 33-52), with 43 (52%) males and 71 (87%) participants of White ethnicity. All participants had detectable spike-specific antibodies following the second dose, and all groups (methotrexate, targeted biologics, and healthy controls) demonstrated similar neutralising antibody titres against wild-type, alpha, and delta variants. By contrast, a lower proportion of participants on methotrexate (eight [62%] of 13, 95% CI 32-86) and targeted biologics (37 [74%] of 50, 60-85; p=0·38) had detectable T-cell responses following the second vaccine dose, compared with controls (14 [100%] of 14, 77-100; p=0·022). There was no difference in the magnitude of T-cell responses between patients receiving methotrexate (median cytokine-secreting cells per 106 cells 160 [IQR 10-625]), targeted biologics (169 [25-503], p=0·56), and controls (185 [133-328], p=0·41).
Interpretation: Functional humoral immunity (ie, neutralising antibody responses) at 14 days following a second dose of BNT162b2 was not impaired by methotrexate or targeted biologics. A proportion of patients on immunosuppression did not have detectable T-cell responses following the second dose. The longevity of vaccine-elicited antibody responses is unknown in this population.
Funding: NIHR Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London; The Psoriasis Association.
© 2022 The Authors. Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Figures

References
-
- MacKenna B, Kennedy NA, Mehkar A, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune modifying therapies: a nationwide cohort study in the OpenSAFELY platform. medRxiv. 2021 doi: 10.1101/2021.09.03.21262888. published online Sep 10. (preprint). - DOI - PMC - PubMed
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

