Translating mouse models of abdominal aortic aneurysm to the translational needs of vascular surgery
- PMID: 34778850
- PMCID: PMC8577080
- DOI: 10.1016/j.jvssci.2021.01.002
Translating mouse models of abdominal aortic aneurysm to the translational needs of vascular surgery
Abstract
Introduction: Abdominal aortic aneurysm (AAA) is a condition that has considerable socioeconomic impact and an eventual rupture is associated with high mortality and morbidity. Despite decades of research, surgical repair remains the treatment of choice and no medical therapy is currently available. Animal models and, in particular, murine models, of AAA are a vital tool for experimental in vivo research. However, each of the different models has individual limitations and provide only partial mimicry of human disease. This narrative review addresses the translational potential of the available mouse models, highlighting unanswered questions from a clinical perspective. It is based on a thorough presentation of the available literature and more than a decade of personal experience, with most of the available models in experimental and translational AAA research.
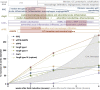
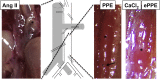
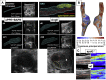
Results: From all the models published, only the four inducible models, namely the angiotensin II model (AngII), the porcine pancreatic elastase perfusion model (PPE), the external periadventitial elastase application (ePPE), and the CaCl2 model have been widely used by different independent research groups. Although the angiotensin II model provides features of dissection and aneurysm formation, the PPE model shows reliable features of human AAA, especially beyond day 7 after induction, but remains technically challenging. The translational value of ePPE as a model and the combination with β-aminopropionitrile to induce rupture and intraluminal thrombus formation is promising, but warrants further mechanistic insights. Finally, the external CaCl2 application is known to produce inflammatory vascular wall thickening. Unmet translational research questions include the origin of AAA development, monitoring aneurysm growth, gender issues, and novel surgical therapies as well as novel nonsurgical therapies.
Conclusion: New imaging techniques, experimental therapeutic alternatives, and endovascular treatment options provide a plethora of research topics to strengthen the individual features of currently available mouse models, creating the possibility of shedding new light on translational research questions.
Keywords: AAA; Abdominal aortic aneurysm; Aneurysm mouse models; Translational research.
© 2021 by the Society for Vascular Surgery. Published by Elsevier Inc.
Figures


References
-
- Oliver-Williams C., Sweeting M.J., Turton G., Parkin D., Cooper D., Rodd C. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68–74. - PubMed
-
- Cosford P.A., Leng G.C. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007:CD002945. - PubMed
-
- Wanhainen A., Verzini F., Van Herzeele I., Allaire E., Bown M., Cohnert T. Editor's choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. - PubMed
-
- Chaikof E.L., Dalman R.L., Eskandari M.K., Jackson B.M., Lee W.A., Mansour M.A. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77 e2. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

