T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study
- PMID: 34778853
- PMCID: PMC8577846
- DOI: 10.1016/S2666-5247(21)00275-5
T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study
Abstract
Background: Previous infection with SARS-CoV-2 affects the immune response to the first dose of the SARS-CoV-2 vaccine. We aimed to compare SARS-CoV-2-specific T-cell and antibody responses in health-care workers with and without previous SARS-CoV-2 infection following a single dose of the BNT162b2 (tozinameran; Pfizer-BioNTech) mRNA vaccine.
Methods: We sampled health-care workers enrolled in the PITCH study across four hospital sites in the UK (Oxford, Liverpool, Newcastle, and Sheffield). All health-care workers aged 18 years or older consenting to participate in this prospective cohort study were included, with no exclusion criteria applied. Blood samples were collected where possible before vaccination and 28 (±7) days following one or two doses (given 3-4 weeks apart) of the BNT162b2 vaccine. Previous infection was determined by a documented SARS-CoV-2-positive RT-PCR result or the presence of positive anti-SARS-CoV-2 nucleocapsid antibodies. We measured spike-specific IgG antibodies and quantified T-cell responses by interferon-γ enzyme-linked immunospot assay in all participants where samples were available at the time of analysis, comparing SARS-CoV-2-naive individuals to those with previous infection.
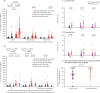
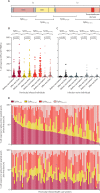
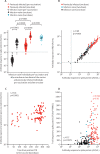
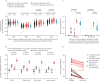
Findings: Between Dec 9, 2020, and Feb 9, 2021, 119 SARS-CoV-2-naive and 145 previously infected health-care workers received one dose, and 25 SARS-CoV-2-naive health-care workers received two doses, of the BNT162b2 vaccine. In previously infected health-care workers, the median time from previous infection to vaccination was 268 days (IQR 232-285). At 28 days (IQR 27-33) after a single dose, the spike-specific T-cell response measured in fresh peripheral blood mononuclear cells (PBMCs) was higher in previously infected (n=76) than in infection-naive (n=45) health-care workers (median 284 [IQR 150-461] vs 55 [IQR 24-132] spot-forming units [SFUs] per 106 PBMCs; p<0·0001). With cryopreserved PBMCs, the T-cell response in previously infected individuals (n=52) after one vaccine dose was equivalent to that of infection-naive individuals (n=19) after receiving two vaccine doses (median 152 [IQR 119-275] vs 162 [104-258] SFUs/106 PBMCs; p=1·00). Anti-spike IgG antibody responses following a single dose in 142 previously infected health-care workers (median 270 373 [IQR 203 461-535 188] antibody units [AU] per mL) were higher than in 111 infection-naive health-care workers following one dose (35 001 [17 099-55 341] AU/mL; p<0·0001) and higher than in 25 infection-naive individuals given two doses (180 904 [108 221-242 467] AU/mL; p<0·0001).
Interpretation: A single dose of the BNT162b2 vaccine is likely to provide greater protection against SARS-CoV-2 infection in individuals with previous SARS-CoV-2 infection, than in SARS-CoV-2-naive individuals, including against variants of concern. Future studies should determine the additional benefit of a second dose on the magnitude and durability of immune responses in individuals vaccinated following infection, alongside evaluation of the impact of extending the interval between vaccine doses.
Funding: UK Department of Health and Social Care, and UK Coronavirus Immunology Consortium.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
CD worked on the Oxford–AstraZeneca COVID-19 vaccine trial (phase 1–3). AO reports personal fees from Take Two Interactive and personal fees from Genome BC, outside the submitted work. PCM reports grants from the Wellcome Trust during the conduct of the study. SLR-J reports grants from the UK Department of Health and Social Care during the conduct of the study and grants from UK Research and Innovation (UKRI), National Institute for Health Research (NIHR), and Global Challenges Research Fund outside the submitted work. SD reports grants from the UK Department of Health and Social Care, UK Coronavirus Immunology Consortium (UKRI), the Huo Family Foundation, and the NIHR during the conduct of the study. CJAD reports grants from the Wellcome Trust during the conduct of the study. LT reports personal fees from Eisai outside the submitted work. All other authors declare no competing interests.
Figures
References
-
- Hall V, Foulkes S, Charlett A, et al. Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? Large multi-centre prospective cohort study (the SIREN study), England: June to November 2020. medRxiv. 2021 doi: 10.1101/2021.01.13.21249642. published online January 15. (preprint) - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 110058/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- FS/18/52/33808/BHF_/British Heart Foundation/United Kingdom
- DH_/Department of Health/United Kingdom
- 211153/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- MR/L006588/1/MRC_/Medical Research Council/United Kingdom
- PG/11/116/29288/BHF_/British Heart Foundation/United Kingdom
- 205228/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 220171/Z/20/Z/WT_/Wellcome Trust/United Kingdom
- MR/W02067X/1/MRC_/Medical Research Council/United Kingdom
- MR/V028448/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

