Transcutaneous Cervical Vagal Nerve Stimulation in Patients with Posttraumatic Stress Disorder (PTSD): A Pilot Study of Effects on PTSD Symptoms and Interleukin-6 Response to Stress
- PMID: 34778863
- PMCID: PMC8580056
- DOI: 10.1016/j.jadr.2021.100190
Transcutaneous Cervical Vagal Nerve Stimulation in Patients with Posttraumatic Stress Disorder (PTSD): A Pilot Study of Effects on PTSD Symptoms and Interleukin-6 Response to Stress
Abstract
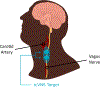
Background: Posttraumatic stress disorder (PTSD) is a highly disabling condition associated with alterations in multiple neurobiological systems, including increases in inflammatory and sympathetic function, responsible for maintenance of symptoms. Treatment options including medications and psychotherapies have limitations. We previously showed that transcutaneous Vagus Nerve Stimulation (tcVNS) blocks inflammatory (interleukin (IL)-6) responses to stress in PTSD. The purpose of this study was to assess the effects of tcVNS on PTSD symptoms and inflammatory responses to stress.
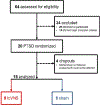
Methods: Twenty patients with PTSD were randomized to double blind active tcVNS (N=9) or sham (N=11) stimulation in conjunction with exposure to personalized traumatic scripts immediately followed by active or sham tcVNS and measurement of IL-6 and other biomarkers of inflammation. Patients then self administered active or sham tcVNS twice daily for three months. PTSD symptoms were measured with the PTSD Checklist (PCL) and the Clinician Administered PTSD Scale (CAPS), clinical improvement with the Clinical Global Index (CGI) and anxiety with the Hamilton Anxiety Scale (Ham-A) at baseline and one-month intervals followed by a repeat of measurement of biomarkers with traumatic scripts. After three months patients self treated with twice daily open label active tcVNS for another three months followed by assessment with the CGI.
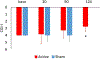
Results: Traumatic scripts increased IL-6 in PTSD patients, an effect that was blocked by tcVNS (p<.05). Active tcVNS treatment for three months resulted in a 31% greater reduction in PTSD symptoms compared to sham treatment as measured by the PCL (p=0.013) as well as hyperarousal symptoms and somatic anxiety measured with the Ham-A p<0.05). IL-6 increased from baseline in sham but not tcVNS. Open label tcVNS resulted in improvements measured with the CGI compared to the sham treatment period p<0.05).
Conclusions: These preliminary results suggest that tcVNS reduces inflammatory responses to stress, which may in part underlie beneficial effects on PTSD symptoms.
Figures


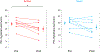

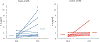
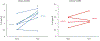
Similar articles
-
Transcutaneous vagal nerve stimulation blocks stress-induced activation of Interleukin-6 and interferon-γ in posttraumatic stress disorder: A double-blind, randomized, sham-controlled trial.Brain Behav Immun Health. 2020 Sep 11;9:100138. doi: 10.1016/j.bbih.2020.100138. eCollection 2020 Dec. Brain Behav Immun Health. 2020. PMID: 34589887 Free PMC article.
-
Transcutaneous cervical vagal nerve stimulation reduces sympathetic responses to stress in posttraumatic stress disorder: A double-blind, randomized, sham controlled trial.Neurobiol Stress. 2020 Oct 20;13:100264. doi: 10.1016/j.ynstr.2020.100264. eCollection 2020 Nov. Neurobiol Stress. 2020. PMID: 33344717 Free PMC article.
-
Effect of transcutaneous cervical vagus nerve stimulation on declarative and working memory in patients with Posttraumatic Stress Disorder (PTSD): A pilot study.J Affect Disord. 2023 Oct 15;339:418-425. doi: 10.1016/j.jad.2023.07.025. Epub 2023 Jul 12. J Affect Disord. 2023. PMID: 37442455 Free PMC article. Clinical Trial.
-
Evaluation of post-traumatic stress disorder (PTSD) and related comorbidities in clinical studies.J Med Life. 2022 Apr;15(4):436-442. doi: 10.25122/jml-2022-0120. J Med Life. 2022. PMID: 35646173 Free PMC article. Review.
-
[Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context].Encephale. 2012 Oct;38(5):373-80. doi: 10.1016/j.encep.2011.12.003. Epub 2012 Jan 24. Encephale. 2012. PMID: 23062450 Review. French.
Cited by
-
Neuromodulation Strategies to Reduce Inflammation and Improve Lung Complications in COVID-19 Patients.Front Neurol. 2022 Jul 14;13:897124. doi: 10.3389/fneur.2022.897124. eCollection 2022. Front Neurol. 2022. PMID: 35911909 Free PMC article. Review.
-
The gut-brain axis and cognitive control: A role for the vagus nerve.Semin Cell Dev Biol. 2024 Mar 15;156:201-209. doi: 10.1016/j.semcdb.2023.02.004. Epub 2023 Feb 16. Semin Cell Dev Biol. 2024. PMID: 36803834 Free PMC article. Review.
-
Acute and long-term effects of COVID-19 on brain and mental health: A narrative review.Brain Behav Immun. 2025 Jan;123:928-945. doi: 10.1016/j.bbi.2024.11.007. Epub 2024 Nov 3. Brain Behav Immun. 2025. PMID: 39500417 Review.
-
Transcutaneous vagal nerve stimulation for the treatment of trauma- and stressor-related disorders: systematic review of randomised controlled studies.BJPsych Open. 2025 Aug 1;11(5):e165. doi: 10.1192/bjo.2025.10057. BJPsych Open. 2025. PMID: 40746136 Free PMC article. Review.
-
Vagus Nerve Stimulation in Stroke Management: Brief Review of Evolution and Present Applications Paired with Rehabilitation.Brain Sci. 2025 Mar 27;15(4):346. doi: 10.3390/brainsci15040346. Brain Sci. 2025. PMID: 40309799 Free PMC article. Review.
References
-
- Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, Reimherr FW, Schwartz TL, Zajecka JM, 2017. A five-year observational study of patients with treatment-resistant depression treated with VNS therapy or treatment-as-usual: comparison of response, remission, and suicidality. Am. J. Psychiatry 174, 640–648. - PubMed
-
- American Psychiatric Association, 2013. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), 5 ed. American Psychiatric Association, Washington, D.C.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
