Prediction of SARS-CoV-2 hosts among Brazilian mammals and new coronavirus transmission chain using evolutionary bioinformatics
- PMID: 34778882
- PMCID: PMC8475823
- DOI: 10.1186/s44149-021-00020-w
Prediction of SARS-CoV-2 hosts among Brazilian mammals and new coronavirus transmission chain using evolutionary bioinformatics
Abstract
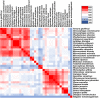
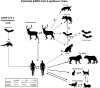
Severe acute respiratory syndrome coronavirus (SARS-CoV) and SARS-CoV-2 are thought to transmit to humans via wild mammals, especially bats. However, evidence for direct bat-to-human transmission is lacking. Involvement of intermediate hosts is considered a reason for SARS-CoV-2 transmission to humans and emergence of outbreak. Large biodiversity is found in tropical territories, such as Brazil. On the similar line, this study aimed to predict potential coronavirus hosts among Brazilian wild mammals based on angiotensin-converting enzyme 2 (ACE2) sequences using evolutionary bioinformatics. Cougar, maned wolf, and bush dogs were predicted as potential hosts for coronavirus. These indigenous carnivores are philogenetically closer to the known SARS-CoV/SARS-CoV-2 hosts and presented low ACE2 divergence. A new coronavirus transmission chain was developed in which white-tailed deer, a susceptible SARS-CoV-2 host, have the central position. Cougar play an important role because of its low divergent ACE2 level in deer and humans. The discovery of these potential coronavirus hosts will be useful for epidemiological surveillance and discovery of interventions that can contribute to break the transmission chain.
Supplementary information: The online version contains supplementary material available at 10.1186/s44149-021-00020-w.
Keywords: Angiotensin-converting enzyme 2; Brazilian mammals; Coronavirus; SARS-CoV-2; White-tailed deer.
© The Author(s) 2021.
Conflict of interest statement
Competing interestsThe authors state that there is no conflict of interest.
Figures

References
-
- Adachi, J., and M. Hasegawa. 1996. Model of amino acid substitution in proteins encoded by mitochondrial DNA. Journal of Molecular Evolution 42 (4): 459–468. 10.1007/BF02498640. - PubMed
-
- Alexander, Matthew R., Clara T. Schoeder, Jacquelyn A. Brown, Charles D. Smart, Chris Moth, John P. Wikswo, John A. Capra, Jens Meiler, Wenbiao Chen, and Meena S. Madhur. 2020. Predicting susceptibility to SARS-CoV-2 infection based on structural differences in ACE2 across species. The FASEB Journal 34 (12): 15946–15960. 10.1096/fj.202001808R. - PMC - PubMed
-
- Beisiegel, Beatriz De Mello, and César Ades. 2002. The behavior of the bush dog (Speothos Venaticus Lund, 1842) in the Field: A review. Revista de Etologia 4 (1): 17–23.
-
- Brandão, Paulo Eduardo, Karin Scheffer, Laura Yaneth Villarreal, Samira Achkar, Rafael de Novaes Oliveira, Willian de Oliveira Fahl, Juliana Galera Castilho, Ivanete Kotait, and Leonardo José Richtzenhain. 2008. A coronavirus detected in the vampire bat Desmodus Rotundus. The Brazilian Journal of Infectious Diseases: An Official Publication of the Brazilian Society of Infectious Diseases 12 (6): 466–468. 10.1590/s1413-86702008000600003. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
