Inactivated Pseudomonas PE(ΔIII) exotoxin fused to neutralizing epitopes of PEDV S proteins produces a specific immune response in mice
- PMID: 34778884
- PMCID: PMC8497069
- DOI: 10.1186/s44149-021-00021-9
Inactivated Pseudomonas PE(ΔIII) exotoxin fused to neutralizing epitopes of PEDV S proteins produces a specific immune response in mice
Abstract
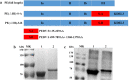
Porcine epidemic diarrhea (PED) caused by the porcine epidemic diarrhea virus (PEDV), is a severe infectious and devastating swine disease that leads to serious economic losses in the swine industry worldwide. An increased number of PED cases caused by variant PEDV have been reported in many countries since 2010. S protein is the main immunogenic protein containing some B-cell epitopes that can induce neutralizing antibodies of PEDV. In this study, the construction, expression and purification of Pseudomonas aeruginosa exotoxin A (PE) without domain III (PEΔIII) as a vector was performed for the delivery of PEDV S-A or S-B. PE(ΔIII) PEDV S-A and PE(ΔIII) PEDV S-B recombinant proteins were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis. The immunogenicity of PEDV S-A and PEDV S-B subunit vaccines were evaluated in mice. The results showed that PEDV-S-B vaccine could not only induce specific humoral and Th1 type-dominant cellular immune responses, but also stimulate PEDV-specific mucosal immune responses in mice. PEDV-S-B subunit vaccine is a novel candidate mucosal vaccine against PEDV infection.
Keywords: Neutralizing antibody; Porcine epidemic diarrhea virus; S protein; Th1-type immune response.
© The Author(s) 2021.
Conflict of interest statement
Competing interestsAuthor Huanchun Chen was not involved in the journal’s review or decisions related to this manuscript. The authors declare no other conflict of interest.
Figures


Similar articles
-
Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus.Microb Cell Fact. 2018 Feb 9;17(1):20. doi: 10.1186/s12934-018-0861-7. Microb Cell Fact. 2018. PMID: 29426335 Free PMC article.
-
Cell Attachment Domains of the Porcine Epidemic Diarrhea Virus Spike Protein Are Key Targets of Neutralizing Antibodies.J Virol. 2017 May 26;91(12):e00273-17. doi: 10.1128/JVI.00273-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381581 Free PMC article.
-
Oral Delivery of Probiotics Expressing Dendritic Cell-Targeting Peptide Fused with Porcine Epidemic Diarrhea Virus COE Antigen: A Promising Vaccine Strategy against PEDV.Viruses. 2017 Oct 25;9(11):312. doi: 10.3390/v9110312. Viruses. 2017. PMID: 29068402 Free PMC article.
-
Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control.Virus Res. 2020 Sep;286:198045. doi: 10.1016/j.virusres.2020.198045. Epub 2020 Jun 2. Virus Res. 2020. PMID: 32502552 Free PMC article. Review.
-
[Advances in reverse genetics to treat porcine epidemic diarrhea virus].Sheng Wu Gong Cheng Xue Bao. 2017 Feb 25;33(2):205-216. doi: 10.13345/j.cjb.160282. Sheng Wu Gong Cheng Xue Bao. 2017. PMID: 28956377 Review. Chinese.
Cited by
-
Development of a multiplex RT-PCR method for the detection of four porcine enteric coronaviruses.Front Vet Sci. 2022 Nov 8;9:1033864. doi: 10.3389/fvets.2022.1033864. eCollection 2022. Front Vet Sci. 2022. PMID: 36425116 Free PMC article.
-
Assessing immunogenicity of CRISPR-NCas9 engineered strain against porcine epidemic diarrhea virus.Appl Microbiol Biotechnol. 2024 Mar 2;108(1):248. doi: 10.1007/s00253-023-12989-0. Appl Microbiol Biotechnol. 2024. PMID: 38430229 Free PMC article.
-
Isolation of porcine epidemic diarrhea virus strain CHCQ-2023 from Chongqing Province and analysis of S gene recombination.BMC Vet Res. 2024 Dec 19;20(1):565. doi: 10.1186/s12917-024-04390-4. BMC Vet Res. 2024. PMID: 39695646 Free PMC article.
-
Research progress of porcine epidemic diarrhea virus S protein.Front Microbiol. 2024 May 30;15:1396894. doi: 10.3389/fmicb.2024.1396894. eCollection 2024. Front Microbiol. 2024. PMID: 38873162 Free PMC article. Review.
-
Prevention and Control of Swine Enteric Coronaviruses in China: A Review of Vaccine Development and Application.Vaccines (Basel). 2023 Dec 21;12(1):11. doi: 10.3390/vaccines12010011. Vaccines (Basel). 2023. PMID: 38276670 Free PMC article. Review.
References
-
- Chen JR, Liao CW, Mao SJT, Weng CN. A recombinant chimera composed of repeat region RR1 of Mycoplasma hyopneumoniae adhesin with Pseudomonas exotoxin: In vivo evaluation of specific IgG response in mice and pigs. Veterinary Microbiology. 2001;80(4):347–357. doi: 10.1016/S0378-1135(01)00315-7. - DOI - PubMed
-
- Chen, P., X. Zhao, S. Zhou, T. Zhou, X. Tan, X. Wu, W. Tong, F. Gao, L. Yu, Y. Jiang, H. Yu, Z. Yang, G. Tong, and Y. Zhou. 2021. A Virulent PEDV Strain FJzz1 with Genomic Mutations and Deletions at the High Passage Level Was Attenuated in Piglets via Serial Passage In Vitro. Virologica Sinica: 1–14. 10.1007/s12250-021-00368-w. - PMC - PubMed
LinkOut - more resources
Full Text Sources
