Drug Resistance, Rather than Low Tenofovir Levels in Blood or Urine, Is Associated with Tenofovir, Emtricitabine, and Efavirenz Failure in Resource-Limited Settings
- PMID: 34779228
- PMCID: PMC9225825
- DOI: 10.1089/AID.2021.0135
Drug Resistance, Rather than Low Tenofovir Levels in Blood or Urine, Is Associated with Tenofovir, Emtricitabine, and Efavirenz Failure in Resource-Limited Settings
Abstract
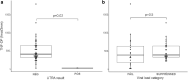
The high cost of viral load (VL) testing limits its use for antiretroviral therapy (ART) adherence support. A low-cost lateral flow urine tenofovir (TFV) rapid assay predicts pre-exposure prophylaxis breakthroughs, but has not yet been investigated in HIV treatment. We therefore evaluated its utility in a pilot cross-sectional study of TFV-containing ART recipients at an increased risk of virologic failure (VF). Participants who had a treatment interruption ≥30 days or had ≥1 episode of viremia (VL ≥400 copies/mL) in the previous year were recruited from a public health setting in Cape Town, South Africa. Self-reported adherence data were collected, the urine TFV assay performed, and concurrent TFV-diphosphate analyzed in dried blood spots. VL testing was done concurrently and, if viremic, genotypic HIV drug resistance testing was performed. Of 48 participants, 18 (37.5%) had VL (>400 copies/mL) at the time of the study, including 16 of 39 receiving efavirenz (EFV), 2 of 6 receiving protease inhibitors, and 0 of 3 receiving dolutegravir. Resistance testing succeeded in 17/18, of which 14 had significant mutations compromising ≥2 agents of the current EFV-based regimen. Of these 14, all had detected urine TFV. Urine TFV was undetectable in two out of three without regimen-relevant resistance; p = .02. In participants on EFV-based regimens returning to care, VF was largely due to viral resistance, where detectable urine TFV had 100% sensitivity (14/14 participants) in predicting resistance. Conversely, when undetectable, the urine-based assay could be used to preclude participants with poor adherence from undergoing costly HIV drug resistance testing.
Keywords: adherence; point of care; real time; resistance; urine.
Conflict of interest statement
All authors have no competing interests, and all work was funded by the NIH/NIAID R01AI152119 and R01AI143340.
Figures
References
-
- The TEMPRANO ANRS 12136 Study Group: A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373:808–822. - PubMed
-
- Rodger AJ, Cambiano V, Phillips AN, et al. : Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet 2019;393:2428–2438. - PMC - PubMed
-
- World Health Organization: Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach, 2nd Ed. World Health Organization, 2016. Available at www.who.int/hiv/pub/arv/arv-2016/en/, accessed February 5, 2016. - PubMed

