Neural control of growth and size in the axolotl limb regenerate
- PMID: 34779399
- PMCID: PMC8716110
- DOI: 10.7554/eLife.68584
Neural control of growth and size in the axolotl limb regenerate
Abstract
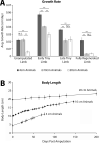
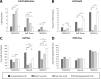
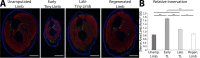
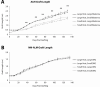
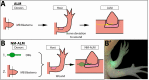
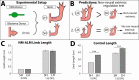
The mechanisms that regulate growth and size of the regenerating limb in tetrapods such as the Mexican axolotl are unknown. Upon the completion of the developmental stages of regeneration, when the regenerative organ known as the blastema completes patterning and differentiation, the limb regenerate is proportionally small in size. It then undergoes a phase of regeneration that we have called the 'tiny-limb' stage, which is defined by rapid growth until the regenerate reaches the proportionally appropriate size. In the current study we have characterized this growth and have found that signaling from the limb nerves is required for its maintenance. Using the regenerative assay known as the accessory limb model (ALM), we have found that growth and size of the limb positively correlates with nerve abundance. We have additionally developed a new regenerative assay called the neural modified-ALM (NM-ALM), which decouples the source of the nerves from the regenerating host environment. Using the NM-ALM we discovered that non-neural extrinsic factors from differently sized host animals do not play a prominent role in determining the size of the regenerating limb. We have also discovered that the regulation of limb size is not autonomously regulated by the limb nerves. Together, these observations show that the limb nerves provide essential cues to regulate ontogenetic allometric growth and the final size of the regenerating limb.
Keywords: axolotl; developmental biology; limb regeneration; neural control; proportionality; regenerative medicine; size regulation; stem cells.
Plain language summary
Humans’ ability to regrow lost or damaged body parts is relatively limited, but some animals, such as the axolotl (a Mexican salamander), can regenerate complex body parts, like legs, many times over their lives. Studying regeneration in these animals could help researchers enhance humans’ abilities to heal. One way to do this is using the Accessory Limb Model (ALM), where scientists wound an axolotl’s leg, and study the additional leg that grows from the wound. The first stage of limb regeneration creates a new leg that has the right structure and shape. The new leg is very small so the next phase involves growing the leg until its size matches the rest of the animal. This phase must be controlled so that the limb stops growing when it reaches the right size, but how this regulation works is unclear. Previous research suggests that the number of nerves in the new leg could be important. Wells et al. used a ALM to study how the size of regenerating limbs is controlled. They found that changing the number of nerves connected to the new leg altered its size, with more nerves leading to a larger leg. Next, Wells et al. created a system that used transplanted nerve bundles of different sizes to grow new legs in different sized axolotls. This showed that the size of the resulting leg is controlled by the number of nerves connecting it to the CNS. Wells et al. also showed that nerves can only control regeneration if they remain connected to the central nervous system. These results explain how size is controlled during limb regeneration in axolotls, highlighting the fact that regrowth is directly controlled by the number of nerves connected to a regenerating leg. Much more work is needed to reveal the details of this process and the signals nerves use to control growth. It will also be important to determine whether this control system is exclusive to axolotls, or whether other animals also use it.
© 2021, Wells et al.
Conflict of interest statement
KW, KK, MB, WV, CM No competing interests declared
Figures




Comment in
-
A matter of nerves.Elife. 2021 Dec 23;10:e75629. doi: 10.7554/eLife.75629. Elife. 2021. PMID: 34939564 Free PMC article.
References
-
- Aizawa R, Yamada A, Suzuki D, Iimura T, Kassai H, Harada T, Tsukasaki M, Yamamoto G, Tachikawa T, Nakao K, Yamamoto M, Yamaguchi A, Aiba A, Kamijo R. Cdc42 is required for chondrogenesis and interdigital programmed cell death during limb development. Mechanisms of Development. 2012;129:38–50. doi: 10.1016/j.mod.2012.02.002. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

