MicroRNA‑190b expression predicts a good prognosis and attenuates the malignant progression of pancreatic cancer by targeting MEF2C and TCF4
- PMID: 34779502
- PMCID: PMC8600408
- DOI: 10.3892/or.2021.8223
MicroRNA‑190b expression predicts a good prognosis and attenuates the malignant progression of pancreatic cancer by targeting MEF2C and TCF4
Abstract
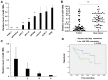
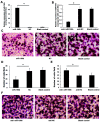
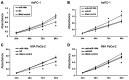
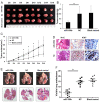
MicroRNAs (miRNAs/miRs) are key components of regulatory networks in cancer. Although miR‑190b is an important tumor‑related miRNA, its role in pancreatic cancer has not been extensively investigated. The aim of the present study was to examine the expression of miR‑190b in pancreatic cancer cell lines and tissues and evaluate its effects on cancer progression. Reverse transcription‑quantitative PCR (RT‑qPCR) analysis was used to measure miR‑190b expression levels in human pancreatic cancer cell lines and tissues, and the association between miR‑190b expression and clinicopathological characteristics was assessed. An in vitro Transwell invasion assay and an in vivo metastasis formation assay were performed using pancreatic cancer cells. The effect of miR‑190b on pancreatic cancer cell proliferation was evaluated using a Cell Counting Kit‑8 assay based on an in vivo xenograft mouse model. The direct targets of miR‑190b were predicted using bioinformatics tools and were validated through western blotting and luciferase reporter assays. Pancreatic cancer cell lines and tissues were found to express lower levels of miR‑190b compared with normal cells and adjacent non‑tumor tissues. Furthermore, high expression of miR‑190b was found to be positively correlated with low T, N and American Joint Committee on Cancer classifications, and predicted a good prognosis. miR‑190b was shown to exert suppressive effects on cancer cell proliferation, invasion and metastasis. In addition, it was also found that miR‑190b directly targeted myocyte enhancer factor 2C (MEF2C) and transcription factor 4 (TCF4) in pancreatic cancer, thus serving as a tumor suppressor and a predictor of good prognosis in pancreatic cancer. The immunohistochemistry and RT‑qPCR results indicated that the MEF2C and TCF4 expression levels were negatively correlated with the miR‑190b expression levels. The findings of the present study highlight the value of miR‑190b as a novel target candidate for pancreatic cancer diagnosis and therapy.
Keywords: microRNA‑190b; myocyte enhancer factor 2C; pancreatic cancer; prognosis; transcription factor 4; tumor suppressor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Candido S, Abrams SL, Steelman LS, Lertpiriyapong K, Martelli AM, Cocco L, Ratti S, Follo MY, Murata RM, Rosalen PL, et al. Effects of the MDM-2 inhibitor Nutlin-3a on PDAC cells containing and lacking WT-TP53 on sensitivity to chemotherapy, signal transduction inhibitors and nutraceuticals. Adv Biol Regul. 2019;72:22–40. doi: 10.1016/j.jbior.2019.03.002. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

