Shuttling of cellular proteins between the plasma membrane and nucleus (Review)
- PMID: 34779504
- PMCID: PMC8600410
- DOI: 10.3892/mmr.2021.12530
Shuttling of cellular proteins between the plasma membrane and nucleus (Review)
Abstract
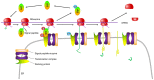
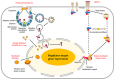
Recently accumulated evidence has indicated that the nucleomembrane shuttling of cellular proteins is common, which provides new insight into the subcellular translocation and biological functions of proteins synthesized in the cytoplasm. The present study aimed to clarify the trafficking of proteins between the plasma membrane and nucleus. These proteins primarily consist of transmembrane receptors, membrane adaptor proteins, adhesive proteins, signal proteins and nuclear proteins, which contribute to proliferation, apoptosis, chemoresistance, adhesion, migration and gene expression. The proteins frequently undergo cross‑talk, such as the interaction of transmembrane proteins with signal proteins. The transmembrane proteins undergo endocytosis, infusion into organelles or proteolysis into soluble forms for import into the nucleus, while nuclear proteins interact with membrane proteins or act as receptors. The nucleocytosolic translocation involves export or import through nuclear membrane pores by importin or exportin. Nuclear proteins generally interact with other transcription factors, and then binding to the promoter for gene expression, while membrane proteins are responsible for signal initiation by binding to other membrane and/or adaptor proteins. Protein translocation occurs in a cell‑specific manner and is closely linked to cellular biological events. The present review aimed to improve understanding of cytosolic protein shuttling between the plasma membrane and nucleus and the associated signaling pathways.
Keywords: nucleus; plasma membrane; signal pathway; subcellular shuttling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures