Empagliflozin, Health Status, and Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The EMPEROR-Preserved Trial
- PMID: 34779658
- PMCID: PMC8763045
- DOI: 10.1161/CIRCULATIONAHA.121.057812
Empagliflozin, Health Status, and Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The EMPEROR-Preserved Trial
Abstract
Background: Patients with heart failure with preserved ejection fraction have significant impairment in health-related quality of life. In the EMPEROR-Preserved trial (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction), we evaluated the efficacy of empagliflozin on health-related quality of life in patients with heart failure with preserved ejection fraction and whether the clinical benefit observed with empagliflozin varies according to baseline health status.
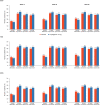
Methods: Health-related quality of life was measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline and 12, 32, and 52 weeks. Patients were divided by baseline KCCQ Clinical Summary Score (CSS) tertiles, and the effect of empagliflozin on outcomes was examined. The effect of empagliflozin on KCCQ-CSS, Total Symptom Score, and Overall Summary Score was evaluated. Responder analyses were performed to compare the odds of improvement and deterioration in KCCQ related to treatment with empagliflozin.
Results: The effect of empagliflozin on reducing the risk of time to cardiovascular death or heart failure hospitalization was consistent across baseline KCCQ-CSS tertiles (hazard ratio, 0.83 [95% CI, 0.69-1.00], 0.70 [95% CI, 0.55-0.88], and 0.82 [95% CI, 0.62-1.08] for scores <62.5, 62.5-83.3, and ≥83.3, respectively; P trend=0.77). Similar results were seen for total heart failure hospitalizations. Patients treated with empagliflozin had significant improvement in KCCQ-CSS versus placebo (+1.03, +1.24, and +1.50 at 12, 32, and 52 weeks, respectively; P<0.01); similar results were seen for Total Symptom Score and Overall Summary Score. At 12 weeks, patients on empagliflozin had higher odds of improvement ≥5 points (odds ratio, 1.23 [95% CI, 1.10-1.37]), ≥10 points (odds ratio, 1.15 [95% CI, 1.03-1.27]), and ≥15 points (odds ratio, 1.13 [95% CI, 1.02-1.26]) and lower odds of deterioration ≥5 points in KCCQ-CSS (odds ratio, 0.85 [95% CI, 0.75-0.97]). A similar pattern was seen at 32 and 52 weeks, and results were consistent for Total Symptom Score and Overall Summary Score.
Conclusions: In patients with heart failure with preserved ejection fraction, empagliflozin reduced the risk for major heart failure outcomes across the range of baseline KCCQ scores. Empagliflozin improved health-related quality of life, an effect that appeared early and was sustained for at least 1 year. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057951.
Keywords: empagliflozin; health status; heart failure, diastolic; quality of life.
Figures




Comment in
-
Designing clinical trials in heart failure with preserved ejection fraction: quality over quantity?Eur J Heart Fail. 2022 May;24(5):851-854. doi: 10.1002/ejhf.2510. Epub 2022 May 5. Eur J Heart Fail. 2022. PMID: 35445788 No abstract available.
References
-
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 - PubMed
-
- Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074 - PubMed
-
- Lewis EF, Lamas GA, O’Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, et al. ; CHARM Investigators. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9:83–91. doi: 10.1016/j.ejheart.2006.10.012 - PubMed
-
- McMurray J, Ostergren J, Pfeffer M, Swedberg K, Granger C, Yusuf S, Held P, Michelson E, Olofsson B; CHARM Committees and Investigators. Clinical features and contemporary management of patients with low and preserved ejection fraction heart failure: baseline characteristics of patients in the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur J Heart Fail. 2003;5:261–270. doi: 10.1016/s1388-9842(03)00052-7 - PubMed
-
- Butler J, Anker SD, Filippatos G, Khan MS, Ferreira JP, Pocock SJ, Giannetti N, Januzzi JL, Piña IL, Lam CSP, et al. ; EMPEROR-Reduced Trial Committees and Investigators. Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021;42:1203–1212. doi: 10.1093/eurheartj/ehaa1007 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

