Control of neurotransmitter release by two distinct membrane-binding faces of the Munc13-1 C1C2B region
- PMID: 34779770
- PMCID: PMC8648301
- DOI: 10.7554/eLife.72030
Control of neurotransmitter release by two distinct membrane-binding faces of the Munc13-1 C1C2B region
Abstract
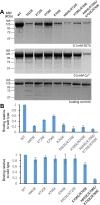
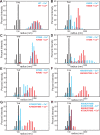
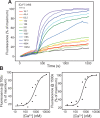
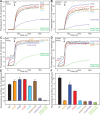
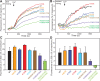
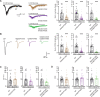
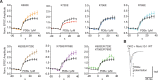
Munc13-1 plays a central role in neurotransmitter release through its conserved C-terminal region, which includes a diacyglycerol (DAG)-binding C1 domain, a Ca2+/PIP2-binding C2B domain, a MUN domain and a C2C domain. Munc13-1 was proposed to bridge synaptic vesicles to the plasma membrane through distinct interactions of the C1C2B region with the plasma membrane: (i) one involving a polybasic face that is expected to yield a perpendicular orientation of Munc13-1 and hinder release; and (ii) another involving the DAG-Ca2+-PIP2-binding face that is predicted to result in a slanted orientation and facilitate release. Here, we have tested this model and investigated the role of the C1C2B region in neurotransmitter release. We find that K603E or R769E point mutations in the polybasic face severely impair Ca2+-independent liposome bridging and fusion in in vitro reconstitution assays, and synaptic vesicle priming in primary murine hippocampal cultures. A K720E mutation in the polybasic face and a K706E mutation in the C2B domain Ca2+-binding loops have milder effects in reconstitution assays and do not affect vesicle priming, but enhance or impair Ca2+-evoked release, respectively. The phenotypes caused by combining these mutations are dominated by the K603E and R769E mutations. Our results show that the C1-C2B region of Munc13-1 plays a central role in vesicle priming and support the notion that two distinct faces of this region control neurotransmitter release and short-term presynaptic plasticity.
Keywords: Munc13; mouse; neuroscience; neurotransmitter release; presynaptic plasticity; synaptic vesicle fusion.
© 2021, Camacho et al.
Conflict of interest statement
MC, BQ, TT, JX, LS, JR, CR No competing interests declared
Figures









References
-
- Best RB, Zhu X, Shim J, Lopes PEM, Mittal J, Feig M. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. Journal of Chemical Theory and Computation. 2012;8:3257–3273. doi: 10.1021/ct300400x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

