Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery
- PMID: 34780067
- PMCID: PMC8592088
- DOI: 10.1002/14651858.CD009286.pub4
Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery
Abstract
Background: Selective serotonin reuptake inhibitors (SSRIs) might theoretically reduce post-stroke disability by direct effects on the brain. This Cochrane Review was first published in 2012 and last updated in 2019.
Objectives: To determine if SSRIs are more effective than placebo or usual care at improving outcomes in people less than 12 months post-stroke, and to determine whether treatment with SSRIs is associated with adverse effects.
Search methods: We searched the Cochrane Stroke Group Trials Register (last searched 7 January 2021), Cochrane Controlled Trials Register (CENTRAL, Issue 7 of 12, 7 January 2021), MEDLINE (1946 to 7 January 2021), Embase (1974 to 7 January 2021), CINAHL (1982 to 7 January 2021), PsycINFO (1985 to 7 January 2021), and AMED (1985 to 7 January 2021). PsycBITE had previously been searched (16 July 2018). We searched clinical trials registers.
Selection criteria: We included randomised controlled trials (RCTs) recruiting stroke survivors within the first year. The intervention was any SSRI, at any dose, for any period, and for any indication. The comparator was usual care or placebo. Studies reporting at least one of our primary (disability score or independence) or secondary outcomes (impairments, depression, anxiety, quality of life, fatigue, cognition, healthcare cost, death, adverse events and leaving the study early) were included in the meta-analysis. The primary analysis included studies at low risk of bias.
Data collection and analysis: We extracted data on demographics, stroke type and, our pre-specified outcomes, and bias sources. Two review authors independently extracted data. We used mean difference (MD) or standardised mean differences (SMDs) for continuous variables, and risk ratios (RRs) for dichotomous variables, with 95% confidence intervals (CIs). We assessed bias risks and applied GRADE criteria.
Main results: We identified 76 eligible studies (13,029 participants); 75 provided data at end of treatment, and of these two provided data at follow-up. Thirty-eight required participants to have depression to enter. The duration, drug, and dose varied. Six studies were at low risk of bias across all domains; all six studies did not need participants to have depression to enter, and all used fluoxetine. Of these six studies, there was little to no difference in disability between groups SMD -0.0; 95% CI -0.05 to 0.05; 5 studies, 5436 participants, high-quality evidence) or in independence (RR 0.98; 95% CI 0.93 to 1.03; 5 studies, 5926 participants; high-quality evidence) at the end of treatment. In the studies at low risk of bias across all domains, SSRIs slightly reduced the average depression score (SMD 0.14 lower, 95% CI 0.19 lower to 0.08 lower; 4 studies; 5356 participants, high-quality evidence) and there was a slight reduction in the proportion with depression (RR 0.75, 95% CI 0.65 to 0.86; 3 studies, 5907 participants, high-quality evidence). Cognition was slightly better in the control group (MD -1.22, 95% CI -2.37 to -0.07; 4 studies, 5373 participants, moderate-quality evidence). Only one study (n = 30) reported neurological deficit score (SMD -0.39, 95% CI -1.12 to 0.33; low-quality evidence). SSRIs resulted in little to no difference in motor deficit (SMD 0.03, -0.02 to 0.08; 6 studies, 5518 participants, moderate-quality evidence). SSRIs slightly increased the proportion leaving the study early (RR 1.57, 95% CI 1.03 to 2.40; 6 studies, 6090 participants, high-quality evidence). SSRIs slightly increased the outcome of a seizure (RR 1.40, 95% CI 1.00 to 1.98; 6 studies, 6080 participants, moderate-quality evidence) and a bone fracture (RR 2.35, 95% CI 1.62 to 3.41; 6 studies, 6080 participants, high-quality evidence). One study at low risk of bias across all domains reported gastrointestinal side effects (RR 1.71, 95% CI 0.33, to 8.83; 1 study, 30 participants). There was no difference in the total number of deaths between SSRI and placebo (RR 1.01, 95% CI 0.82 to 1.24; 6 studies, 6090 participants, moderate quality evidence). SSRIs probably result in little to no difference in fatigue (MD -0.06; 95% CI -1.24 to 1.11; 4 studies, 5524 participants, moderate-quality of evidence), nor in quality of life (MD 0.00; 95% CI -0.02 to 0.02, 3 studies, 5482 participants, high-quality evidence). When all studies, irrespective of risk of bias, were included, SSRIs reduced disability scores but not the proportion independent. There was insufficient data to perform a meta-analysis of outcomes at end of follow-up. Several small ongoing studies are unlikely to alter conclusions.
Authors' conclusions: There is high-quality evidence that SSRIs do not make a difference to disability or independence after stroke compared to placebo or usual care, reduced the risk of future depression, increased bone fractures and probably increased seizure risk.
Trial registration: ClinicalTrials.gov NCT02683213.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Lynn A Legg: none known.
Ann‐Sofie Rudberg: none known.
Xing Hua: none known.
Simiao Wu: none known.
Maree L Hackett:
Russel Tilney: none known.
Linnea Lindgren: none known.
Mansur A Kutlubaev: none known.
Cheng‐Fang Hsieh: none known.
Amanda Barugh: none known.
Graeme J Hankey:
Erik Lundström:
Martin Dennis:
Gillian E Mead:
Gillian Mead, Martin Dennis, Maree Hackett, Erik Lundstrom and Graeme Hankey are investigators on the FOCUS trial (Fluoxetine or control under supervision) in the UK, the AFFINITY (Assessment of fluoxetine in stroke recovery) trial in Australia, and the EFFECTs trial in Sweden designed to assess the impact of fluoxetine on disability and dependency after stroke. None of these review authors extracted data from these three trials.
Figures
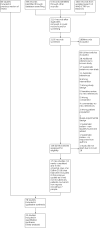




























Update of
-
Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery.Cochrane Database Syst Rev. 2019 Nov 26;2019(11):CD009286. doi: 10.1002/14651858.CD009286.pub3. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 15;11:CD009286. doi: 10.1002/14651858.CD009286.pub4. PMID: 31769878 Free PMC article. Updated.
References
References to studies included in this review
Acler 2009 {published data only}
-
- Acler M, Avesani A, Fiaschi A, Manganotti P. Serotonergic modulation of brain excitability and motor recovery in patients affected by stroke. A double blind placebo RCT. International Journal of Stroke 2008;3:336.
-
- Acler M, Robol E, Fiaschi A, Manganotti P. A double blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. Journal of Neurology 2009;256(7):1152-8. - PubMed
AFFINITY 2020 {published data only}
-
- ACTRN12611000774921. Assessment of fluoxetine in stroke recovery (AFFINITY) trial, 2011. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000774921 (first received 22 July 2011).
-
- ACTRN12619000986178. The assessment of fluoxetine in stroke recovery imaging and blood biomarkers sub-study AFFINITY BM. www.anzctr.org.au/Trial/Registration/TrialReview.aspx ?ACTRN=12619000986178 (first received 11 July 2019).
-
- AFFINITY Trial Collaboration. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurology 2020;19(8):651-60. - PubMed
-
- Hankey G. Assessment oF FluoxetINe In sTroke recoverY (AFFINITY). www.affinitytrial.org/ (accessed 19th February 2019).
Almeida 2006 {published data only}
-
- Almeida OP, Waterreus A, Hankey GJ. Preventing depression after stroke: results from a randomized placebo-controlled trial. Journal of Clinical Psychiatry 2006;67(7):1104-9. - PubMed
Andersen 1994 {published data only}
-
- Andersen G, Vestergaard K, Lauritzen L. Effective treatment of post-stroke depression with selective serotonin reuptake inhibitors. Journal of Neurology 1994;241:S42. - PubMed
-
- Andersen G, Vestergaard K, Lauritzen L. Effective treatment of post-stroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke 1994;25(6):1099-104. - PubMed
-
- Andersen G, Vestergaard K, Lauritzen L. Post-stroke depression treated with citalopram. Acta Neurologica Scandinavica 1994;89 Suppl 155:20.
-
- Andersen G, Vestergaard K, Lauritzen L. Post-stroke depression treated with citalopram - a selective serotonin reuptake inhibitor. Canadian Journal of Neurological Sciences 1993;20:S115.
-
- Andersen G, Vestergaard K, Lauritzen L. Post-stroke depression treated with citalopram a selective serotonin reuptake inhibitor. In: 7th Scandinavian Meeting on Cerebrovascular Disease. 1993:54.
Andersen 2013 {published data only}
-
- Andersen G. The efficacy of citalopram treatment in acute stroke (TALOS). www.clinicaltrials.gov/ct2/show/NCT01937182 (first received 9 September 2013).
-
- Damsbo AG, Mortensen JK, Kraglund KL, Johnsen SP, Andersen G, Blauenfeldt RA. Prestroke physical activity and poststroke cognitive performance. Cerebrovascular Diseases 2020;49(6):632-8. - PubMed
-
- Kraglund KL, Mortensen JK, Damsbo AG, Modrau B, Simonsen SA, Iversen, HK, et al. Neuroregeneration and vascular protection by citalopram in acute ischemic stroke (TALOS). Stroke 2018;49(11):2568-76. - PubMed
-
- Kraglund KL, Mortensen JK, Damsbo AG, Modrau B, Simonsen SA, Iversen HK, et al. Neuroregeneration and vascular protection by citalopramin in acute ischemic stroke (TALOS). Stroke 2018;49(11):2568-76. - PubMed
-
- Kraglund KL, Mortensen JK, Grove EL, Johnsen SP, Andersen G. TALOS: a multicenter, randomised, double blind, placebo controlled trial to test the effects of citalopram in patients with acute stroke; protocol. International Journal of Stroke 2015;10:985-87. - PubMed
Asadollahi 2018 {published data only}
-
- Asadollahi M, Ramezani M, Khanmoradi Z, Karimialavijeh E. The efficacy comparison of citalopram, fluoxetine, and placebo on motor recovery after ischemic stroke: a double-blind placebo-controlled randomized controlled trial. Clinical Rehabilitation 2018;32(8):1069-75. - PubMed
Bembenek 2020 {published data only}
-
- Bembenek JP, Niewada M, Klysz B, Mazur A, Kurczych K, Gluszkiewicz M, et al. Fluoxetine for stroke recovery improvement - the doubleblind, randomised placebo-controlled FOCUS-Poland trial. Neurologia i Neurochirurgia Polska 2020;54(6):544-51. - PubMed
-
- Fluoxetine or control under supervision: Poland. Polish Office for Registration of Medicinal Products, Medical Devices and Biocidal Products - EduraCT No: 2014-001335-37 2014.
Birchenall 2019 {published and unpublished data}
-
- Birchenall J, Térémetz M, Roca P, Lamy JC, Oppenheim C, Maier MA, et al. Individual recovery profiles of manual dexterity, and relation to corticospinal lesion load and excitability after stroke - a longitudinal pilot study. Neurophysiologie Clinique 2019;49(2):149-64. - PubMed
-
- NCT02063425. Evaluation by transcranial magnetic stimulation of the benefit of fluoxetine on motor recovery after stroke (EFLUSTIM). clinicaltrials.gov/ct2/show/NCT02063425 (first received 14 February 2014).
Brown 1998 {published data only}
-
- Brown KW, Sloan RL, Pentland B. Fluoxetine as a treatment for post-stroke emotionalism. Acta Psychiatrica Scandinavica 1998;98(6):455-8. - PubMed
Burns 1999 {published data only}
-
- Burns A, Russell E, Stratton-Powell H, Tyrell P, O'Neill P, Baldwin R. Sertraline in stroke-associated lability of mood. International Journal of Geriatric Psychiatry 1999;14(8):681-5. - PubMed
Cao 2020 {published data only}
-
- Cao JX, Liu L, Sun YT, Zeng QH, Yang ZD, Chen JC. Escitalopram improves neural functional prognosis and endothelial dysfunction in patients with acute cerebral infarction. Restorative Neurology and Neuroscience 2020;38(5):385-93. - PubMed
Chen 2001 {published data only}
-
- Chen WY, Liu FY, Yang AP. Study of effect of integrative Chinese herbs with fluoxetine on rehabilitation of neurological impairment in patients with post-stroke depression. Journal of Chengdu University of Traditional Chinese Medicine 2001;24(4):20-3.
Chen 2002 {published data only}
-
- Chen W, Wang G-F, Chen X-H, Sheng Y-L, Zhu H. Effects of paroxetine on function recovery in patients with post-stroke depression. Chinese Journal of Clinical Rehabilitation 2002;6(13):2014-5.
Chen 2005a {published data only}
-
- Chen KN. Changes of neurotransmitter in patients with post-stroke depression observed with encephalofluctuography technology. Chinese Journal of Clinical Rehabilitation 2005;9(16):118-9.
Chen 2005b {published data only}
-
- Chen T, Li J, Han M. A study on paroxetine in the treatment for post-stroke depression. Jiangxi Medicine 2005;40(7):382-4.
Chen 2015 {published data only}
-
- Chen Z, Xu X, Wu Y, Huang L. Clinical efficacy of drug intervention in patients with acute depression after stroke. International Journal of Psychiatry 2015;42(5):97-100.
Cheng 2003 {published data only}
-
- Cheng F, Shao G, Bao S. Study of effect on neurologic function rehabilitation in patient with post-stroke depression. Chinese Journal of Clinical Rehabilitation 2003;7(1):108-9.
Chollet 2011 {published data only}
-
- Chollet F, Tardy J, Albucher J-F, Thalamas E, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurology 2011;10(2):123-30. - PubMed
Dam 1996 {published data only}
-
- Dam M, Tonin P, De Boni A, Pizzolato G, Casson S, Ermani M, et al. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke 1996;27(7):1211-4. - PubMed
Dike 2019 {published data only}
-
- Dike F. Pharmacological enhancement of motor function recovery in patients with ischaemic stroke: a trial of fluoxetine. www.pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=967 (last accessed 16 February 2021).
-
- Dike FO, Ekeh BC, Ogun AS, Ekrikpo EU, Walshak P, Ogunniy AA. Pharmacological enhancement of motor function recovery in patients with ischaemic stroke: a trial of fluoxetine. Journal of Neurology and Stroke 2019;9(1):47-51.
EFFECTS 2020 {published data only}
-
- EFFECTS Trial Collaboration. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurology 2020;19(8):661-9. - PubMed
-
- ISRCTN13020412. Establishing the effect(s) and safety of fluoxetine initiated in the acute phase of stroke. www.isrctn.com/ISRCTN13020412 (first received 19 December 2014).
-
- Lundstrom E, Isaksson E, Nasman P, Wester P, Martensson B, Norrving B, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurology 2020;19(8):661‐9. - PubMed
Feng 2004 {published data only}
-
- Feng B-L, Wang Q-C, Zheng-Yuan LI. Influence of Jieyu Huoxue decoction on rehabilitation of patients with depression after cerebral infarction. Journal of Chinese Integrated Medicine 2004;2(3):182-4. - PubMed
FOCUS 2019 {published data only}
-
- Anonymous. The FOCUS (Fluoxetine or Control under Supervision) trial results: effects of a 6 month course of fluoxetine on the Stroke Impact Scale, mood and fatigue in patients with stroke. www.ed.ac.uk/clinical-brain-sciences/research/completed-studies-trials/f... (accessed 12 02 2021).
-
- Cook R, Thomas V, Martin R. Does fluoxetine improve recovery after stroke? BMJ 2019;364:1029. - PubMed
-
- Dennis M, Forbes J, Graham C, Hackett ML, Hankey GJ, House A, et al. Fluoxetine and fractures after stroke: exploratory analyses from the FOCUS Trial. Stroke 2019;50(11):3280-2. - PubMed
Fruehwald 2003 {published data only}
-
- Fruehwald S, Gatterbauer E, Rehak P, Baumhackl U. Early fluoxetine treatment of post-stroke depression: a three-month double-blind placebo-controlled study with an open-label long-term follow up. Journal of Neurology 2003;250(3):347-51. - PubMed
Gao 2017 {published data only}
-
- Gao J, Lin M, Zhao J, Bi S, Ni Z, Shang X. Different interventions for post-ischaemic stroke depression in different time periods: a single-blind randomized controlled trial with stratification by time after stroke. Clinical Rehabilitation 2017;31(7):71-81. - PubMed
GlaxoSmithKline 1998 {published data only}
-
- GlaxoSmithKline. An 8-week double-blind placebo controlled parallel group study to assess the efficacy and tolerability of paroxetine in patients suffering from depression following stroke. GSK Clinical Study Register www.gsk clinicalstudyregister.com (accessed 31 August 2012).
Gong 2020 {published data only}
-
- Gong L, Yang X, Feng Y, Fei Z, Wang M, Qin B, et al. The efficacy of integrative anti-depressive therapy on motor recovery after ischemic stroke: a randomized clinical trial. European Journal of Integrative Medicine 2020;35:101102.
Guo 2009 {published data only}
-
- Guo R-Y, Su L, Wang CX. Effects of Linggui Bafa on the therapeutic effect and quality of life in patients of post-stroke depression. Chinese Acupuncture & Moxibustion 2009;29(10):785-90. - PubMed
He 2004 {published data only}
-
- He P. Randomized controlled observation on the effect of early application of fluoxetine in preventing depression after stroke. Chinese Journal of Clinical Rehabilitation 2004;8(28):6016-7.
He 2005 {published data only}
-
- He Y, Wang X, Xiao C. Prospective study of effects of paroxetine with mental intervention on depression and anxiety after stroke. Nervous Diseases and Mental Health 2005;5(1):6-13.
-
- Wang X, He Y, Xiao C-L. A clinical trial of paroxetine and psychotherapy in patients with post-stroke depression and anxiety. Chinese Mental Health Journal 2005;19:564-6.
He 2016 {published data only}
-
- ChiCTR-TRC-12002078. Multi-center randomized clinical study of antidepressant treatment (fluoxetine) on secondary prevention of ischemic stroke. www.chictr.org.cn/showprojen.aspx?proj=7471 (first received 3 April 2012).
-
- Guo Y, He Y, Tang B, Ma K, Cai Z, Zeng S, et al. Effect of using fluoxetine at different time windows on neurological functional prognosis after ischaemic stroke. Restorative Neurology and Neuroscience 2016;34:177-87. - PubMed
-
- He Y, Cai Z, Zeng S, Chen S, Tang B, Liang Y, et al. Effect of fluoxetine on three-year recurrence in acute ischemic stroke: a randomized controlled clinical study. Clinical Neurology and Neurosurgery 2018;168:1-6. - PubMed
-
- He Y-T, Tang B-S, Cai Z-L, Zeng S-L, Jiang X, Guo Yi. Effects of fluoxetine on neural functional prognosis after ischemic stroke: a randomized controlled study in China. Journal of Stroke and Cerebrovascular Diseases 2016;25(4):761-70. - PubMed
Hu 2002 {published data only}
-
- Hu Y, Suo A, Xiang L. The comparative study of the effectiveness of fluoxetine on the stroke patients with depressive symptoms. Shanghai Archives of Psychiatry 2002;14:149-50.
Hu 2018 {published data only}
-
- Hu H, Wang M. Effects of escitalopram on post-stroke depression and cognitive function of patients with neurological function. Hebei Medicine 2018;24(2):325-8.
Huang 2002 {published data only}
-
- Huang X-H. The clinical correlation study and the effect of fluoxetine intervention on poststroke depression. Chinese Journal of Clinical Rehabilitation 2002;6(15):2296-7.
Jia 2005 {published data only}
-
- Jia W. Effect of early intervention on recovery of motor function and recurrent stroke in patients with post-stroke depression. Chinese Journal of Clinical Rehabilitation 2005;9(12):4-5.
Kim 2017 {published data only}
-
- Kim JS, Lee E-J, Chang D-ll, Park J-H, Ahn SH, Cha J-K, et al. Efficacy of early administration of escitalopram on depressive and emotional symptoms and neurological dysfunction after stroke: a multicentre, double-blind, randomised, placebo-controlled study. Lancet Psychiatry 2017;4(1):33-41. - PubMed
-
- Lee EJ, Kim JS, Chang DI, Park JH, Ahn SH, Cha JK, et al. Post-stroke depressive symptoms: varying responses to escitalopram by individual symptoms and lesion location. Journal of Geriatric Psychiatry and Neurology 2020;10:891988720957108. - PubMed
-
- Lee EJ, Oh MS, Kim JS, Chang DI, Park JH, Cha JK, EMOTION investigators. Serotonin transporter gene polymorphisms may be associated with poststroke neurological recovery after escitalopram use. Journal of Neurology, Neurosurgery and Psychiatry 2018;89(3):271-6. - PubMed
-
- NCT01278498. The preventative effect of escitalopram on depression and related emotional disorders in acute stroke patients, 2011. clinicaltrials.gov/ct2/show/NCT01278498 (first received 19 January 2011). [NCT01278498]
Kong 2007 {published data only}
-
- Kong Y. Fluoxetine for poststroke depression: a randomized placebo controlled clinical trial. Neural Regeneration Research 2007;2(3):162-5.
Lai 2006 {published data only}
-
- Lai J. The effect of using paroxetine to treat post stroke depression. Journal of Guangdong Medical College 2006;24(6):585-6.
Li 2004a {published data only}
-
- Li J, He Q-Y, Han M-F. Recent effect of fluoxetine in improving neurologic impairment and preventing post-stroke depression in the early stage. Chinese Journal of Clinical Rehabilitation 2004;8(7):1208-9.
Li 2004b {published data only}
-
- Li C-M, Jiang X-D, Liao G, Lei J-M, Lan S, Ni F-W. Effect of antidepressant drugs in early period on the recovery of post-stroke depression. Chinese Journal of Clinical Rehabilitation 2004;8(19):3713-5.
Li 2005 {published data only}
-
- Li Y, Wang X, Qian FS. Related factors of post-stroke depression and effect of paroxetine. Shandong Archives of Psychiatry 2005;18(4):209-10.
Li 2006 {published data only}
-
- Li W-Q, Li D-X. The efficacy of citalopram for post-stroke depression and its effects on stroke rehabilitation. International Journal of Cerebrovascular Diseases 2006;14(4):275-8.
Li 2007 {published data only}
-
- Li Z. Clinical efficacy of paroxetine in the treatment of post-stroke depression. Modern Journal of Integrated Traditional Chinese and Western Medicine 2007;16(34):5103-4.
Li 2008 {published data only}
-
- Li L-T, Wang S-H, Ge H-Y, Chen J, Yue S-W, Yu M. The beneficial effects of the herbal medicine free and easy wanderer plus (FEWP) and fluoxetine on post-stroke depression. Journal of Alternative and Complementary Medicine 2008;14(7):841-6. - PubMed
Li 2017 {published data only}
-
- Li J, Wang J, Li Y, Zhang W, Zhao J. Effects of escitalopram oxalate on post-stroke depression, cognition and neurological function. Journal of Hebei Medical University 2017;38(5):589-92.
Liu 2006 {published data only}
-
- Liu Y, Xu R. Effect of citalopram treatment on post-stroke depression and neurological functional rehabilitation. Chinese Journal of Rehabilitation 2006;21(3):174-5.
Marquez Romero 2013 {published data only}
-
- Marquez-Romero JM, Arauz A, Ruiz-Sandoval JL, Cruz-Estrada Ede L, Huerta-Franco MR, Aguayo-Leytte G, et al. Fluoxetine for motor recovery after acute intracerebral hemorrhage (FMRICH): study protocol for a randomized, double-blind, placebo-controlled, multicenter trial. Trials 2013;14:77. - PMC - PubMed
-
- Marquez-Romero JM, Reyes-Martínez M, Huerta-Franco MR, Ruiz-Franco A, Silos H, Arauz A. Fluoxetine for motor recovery after acute intracerebral hemorrhage, the FMRICH trial. Clinical Neurology and Neurosurgery 2020;190:105656. - PubMed
-
- Marquez-Romero JM, Reyes-Martínez M, Huerta-Franco MR, Ruiz-Franco A, Silos H, Arauz A. Fluoxetine for motor recovery after acute intracerebral hemorrhage, the FMRICH Trial. Correspondence from Marquez-Romero 2018. - PubMed
-
- Marquez-Romero JM. Fluoxetine for motor recovery after acute intracerebral hemorrhage (FMRICH). www.clinicaltrials.gov/ct2/show/NCT01737541 (first received 29 November 2012).
Meara 1998 {published data only}
-
- Meara RJ, Thalanany M, Balonwu V, Hobson P. The treatment of depression after stroke with the selective serotonin reuptake inhibitor sertraline. Cerebrovascular Diseases 1998;8 Suppl 4:90.
Miao 2004 {published data only}
-
- Miao S-Y, Shi Y-J. Related factors of post-stroke depression and therapeutical effect of citalopram. Chinese Journal of Clinical Rehabilitation 2004;8(19):3718-9.
Murray 2005 {published data only}
-
- Murray V, Von Arbin M, Bartfai A, Berggren A-L, Landtblom A-M, Lumdmark J, et al. Double-blind comparison of sertraline and placebo in stroke patients with minor depression and less severe major depression. Journal of Clinical Psychiatry 2005;66(6):708-16. - PubMed
NCT00177424 {published data only}
-
- NCT00177424. Setraline for prevention post stroke depression and improving rehabilitation outcomes. clinicaltrials.gov/ct2/show/NCT00177424 (first received 15 September 2005).
NCT01674868 {published data only}
-
- NCT01674868. Fluoxetine for motor, aphasia, and neglect recovery after ischemic stroke (FLAN). www.clinicaltrials.gov/ct2/show/NCT01674868 (first received 29 August 2012).
NCT02737930 {published data only}
-
- NCT02737930. Fluoxetine for visual recovery after ischaemic stroke (FLUORESCE). www.clinicaltrials.gov/ct2/show/NCT02737930 (first received 14 April 2016).
Pan 2018 {published data only}
-
- Pan X-L, Chen H-F, Cheng X, Hu C-C, Wang J-W, Fu Y-M, et al. Effects of paroxetine on motor and cognitive function recovery in patients with non-depressed ischemic stroke: an open randomized controlled study. Brain Impairment;doi: 10.1017/BrImp.2018.6.
Pariente 2001 {published data only}
-
- Guiraud-Chaumeil B, Pariente J, Albucher J-F, Loubinoux I, Chollet F. Rehabilitation after stroke [Recuperation neurologique post-ischemique]. Bulletin de l'Académie Nationale de Médecine 2002;6:1015-24. - PubMed
-
- Pariente J, Loubinoux I, Carel C, Albucher JF, Leger A, Manelfe C, et al. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Annals of Neurology 2001;50(6):718-29. - PubMed
Rasmussen 2003 {published data only}
-
- Rasmussen A, Lunde M, Poulsen D, Sørensen K, Qvitzau S, Bech P. A double-blind placebo controlled study of sertraline in the prevention of depression in stroke patients. European Neuropyschopharmacology 2002;12:231. - PubMed
-
- Rasmussen A, Lunde M, Poulsen DL, Sørensen K, Qvitzau S, Bech P. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics 2003;44(3):216-22. - PubMed
-
- Rasmussen A. Depression and stroke. Nordic Journal of Psychiatry 2001;55(4):288.
-
- Rasmussen A. Prophylactic treatment for post-stroke depression and comorbidity. Journal of Psychosomatic Research 2000;48(3):66.
Razazian 2014 {published data only}
-
- Razazian N, Esmaeili O, Almasi A. Effect of fluoxetine on motor improvement in ischemic stroke patients: a double blind clinical trial study. Zahedan Journal of Research in Medical Sciences 2016;18(7):e75492016.
-
- Razazian N. A survey for assessment of effectiveness of fluoxetine on motor improvement in ischemic stroke patients. www.en.irct.ir/trial/8797 (first received 9 January 2014).
Restifo 2001 {published data only}
-
- Restifo DA, Lo Prest R, Lanza S, Giuffrida S, D'Aleo G, Rifici Di Bella C, et al. Motor cortex reorganization induced by fluoxetine in poststroke hemiplegic patients undergoing rehabilitation therapy: a study with transcranial magnetic stimulation. Neurorehabilitation and Neural Repair 2001;15(4):284.
Robinson 2000a {published data only}
-
- Jorge RE, Robinson RG, Arndt S, Starkstein S. Mortality and post-stroke depression: a placebo controlled trial of antidepressants. American Journal of Psychiatry 2003;160:1823-9. - PubMed
-
- Narushima K, Kosier JT, Robinson RG. Preventing post-stroke depression: a 12 week double blind randomised treatment trial and 21 month follow-up. Journal of Nervous and Mental Diseases 2002;190:296-303. - PubMed
-
- Robinson RG, Schultz SK, Castillo C, Kopel T, Kosier JT, Newman RM, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. American Journal of Psychiatry 2000;157(3):351-9. - PubMed
Robinson 2000b {published data only}
-
- Jorge RE, Robinson RG, Arndt S, Starkstein S. Mortality and post-stroke depression: a placebo controlled trial of antidepressants. American Journal of Psychiatry 2003;160:1823-9. - PubMed
-
- Narushima K, Robinson RG. Preventing post-stroke depression: a 12 week double blind randomised treatment trial and 21 month follow-up. Journal of Nervous and Mental Diseases 2002;190:296-303. - PubMed
-
- Robinson RG, Schultz SK, Castillo C, Kopel T, Kosier JT, Newman RM, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. American Journal of Psychiatry 2000;157(3):351-9. - PubMed
Robinson 2008 {published data only}
-
- Robinson RG, Arndt S. Incomplete financial disclosure in a study of escitalopram and problem solving therapy for prevention of post-stroke depression. JAMA 2009;301:1023-4. - PubMed
Savadi Oskouie 2012 {published data only}
-
- Savadi Oskouie D, Sharifipour E, Sadeghi Bazargani H, Hashemilar M, Nikanfar M, Ghazanfari Amlashi S, et al. Efficacy of citalopram on acute ischemic stroke outcome: a randomized clinical trial. Neurorehabilitation and Neural Repair 2017;31(7):638-47. - PubMed
-
- Savadi Oskouie D. Evaluation of the effect of citalopram on three months functional prognosis of acute ischemic stroke patients: a randomized clinical trial. www.en.irct.ir/trial/1766 (first received 22 April 2012).
Shah 2016 {published data only}
-
- Shah IA, Asimi RP, Kawoos Y, Wani MA, Wani MA, Dar MA. Effect of fluoxetine on motor recovery after acute haemorrhagic stroke: a randomized trial. Journal of Neurology and Neurophysiology 2016;7(2). [DOI: 10.4172/2155-9562.1000364] - DOI
Song 2006 {published data only}
-
- Song J-G. Effects of fluoxetine hydrochloride on depressive symptoms and P300 after cerebral stroke. Chinese Journal of Clinical Rehabilitation 2006;10(14):160-2.
Wang 2003 {published data only}
-
- Wang X, Tan Z, Wu Z, Gao J, Feng M. The effects of anti-depression therapy on post-stroke depression and neurologic rehabilitation in the elderly patients. Chinese Journal of Geriatrics 2003;22(5):270-3.
Wang 2009 {published data only}
-
- Wang X. The efficacy of paroxetine in the treatment of post-stroke depression in 55 cases. Chinese Community Physician (Medical Professional Half-monthly) 2009;11(7):11.
Wen 2006 {published data only}
-
- Wen Z-X. The influence of post-stroke prophylactic anti-depression treatment on nerve functional rehabilitation. Acta Academiae Medicinae Qingdao Universitatis 2006;42(3):253-4.
Wiart 2000 {published data only}
-
- Wiart L, Petit H, Joseph PA, Mazaux JM, Barat M. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke 2000;31(8):1829-32. - PubMed
Xie 2005 {published data only}
-
- Xie R, Liu J, Quan H. A prospective random clinical contrast study of treatment with sertraline in elderly patients with post-stroke depression. Chinese Journal of Clinical Neuroscience 2005;13(3):294-7.
Xu 2001 {published data only}
-
- Xu J, Tan J, Ou L. A study on treatment of fluoxetine to depression in early recovery stage of cerebral infarction. Chinese Journal of Rehabilitation Medicine 2001;16(5):281-3.
Xu 2006 {published data only}
-
- Xu J, Wang J, Liu J. Preventive effects of antidepressants on post-stroke depression. Chinese Mental Health Journal 2006;20(3):186-8.
Yang 2002 {published data only}
-
- Yang J, Zhao Y, Bai S. Controlled study on antidepressant treatment of patients with post-stroke depression. Chinese Mental Health Journal 2002;16(12):871-2.
Yang 2011 {published data only}
-
- Yang J. Therapeutic effect of paroxetine on patients with early poststroke depression and the serum interleukins. Chinese Journal of Cerebrovascular Diseases 2011;8(5):235-8.
Ye 2004 {published data only}
-
- Ye L-X, Wang H, Wang Y-D, Zhong L, Liang D-S, Guo Y. Effect of anti-depressive therapy on the rehabilitation of psychological and neurological function after stroke. Chinese Journal of Clinical Rehabilitation 2004;8(31):6826-8.
-
- Ye LX. Effect of paxil and berhomine on post-stroke anxiety-depression and neurological recovery. Chinese Journal of Clinical Rehabilitation 2006;10(6):153-5.
Zhao 2011 {published data only}
-
- Zhao P, Wang J-P. Effects of antidepressants on neurofunctional recovery of post-stroke patients with aphasia. Journal of Dalian Medical University 2011;33(1):55-7.
Zhou 2008 {published data only}
-
- Zhou Z-l, Liang L-Z, Yan Y-X. Preventive effects of fluoxetine on post-stroke depression. Chinese Journal of Modern Applied Pharmacy 2008;25(3):263-4.
References to studies excluded from this review
ACTRN12619000573156 {published data only}
-
- ACTRN12619000573156. FRAMBOISE: Fluoxetine, Recovery And Motor BiOmarkers In StrokE. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=377306 (first received 11 April 2019).
Andersen 1993 {published data only}
-
- Andersen G, Vestergaard K, Riis JO. Citalopram for post-stroke pathological crying. Lancet 1993;342(8875):837-9. - PubMed
Andersen 2012 {published data only}
-
- Andersen G. Efficacy of escitalopram treatment in acute stroke and the role of specific genotypes in stroke. www.clinicaltrialsregister.eu/ctr-search/search?query=2011-005541-12 (first received 27 February 2012).
-
- Andersen G. Escitalopram Treatment In Acute Stroke (ESTIAS). www.clinicaltrials.gov/ct2/show/NCT01561092 (first received 22 March 2012).
Anderson 2002 {published data only}
-
- Anderson C, Hackett M, Carter K, Ni MC. Maximising outcome by overcoming depression in stroke (MOODS) trial. Cerebrovascular Diseases 2002 2002;13 Suppl 3:79.
Anonymous 2012a {published data only}
-
- Anonymous 2012. Stroke and depression. Johns Hopkins Medical Letter: Health After 50 2012;23(11):3. - PubMed
Anonymous 2012b {published data only}
-
- Anonymous. Drug & Device News. Medical Malpractice Law & Strategy, New York Law Journal Newsletters 2012;30(2):5.
Berends 2009 {published data only}
-
- Berends HI, Nijlant JM, Putten MJ, Movig KL, IJzerman MJ. Single dose of fluoxetine increases muscle activation in chronic stroke patients. Clinical Neuropharmacology 2009;32(1):1-5. [also known as Flu 2006] - PubMed
Bonin Pinto 2019 {published data only}
-
- Bonin Pinto C, Morales-Quezada L, Toledo Piza PV, Zeng D, Saleh Vélez FG, Ferreira IS, et al. Combining fluoxetine and rTMS in poststroke motor recovery: a placebo-controlled double-blind randomized phase 2 clinical trial. Neurorehabilitation and Neural Repair 2019;33:643-55. [DOI: 10.1177/1545968319860483.] - DOI - PMC - PubMed
-
- NCT02208466. Effects rTMS combined with fluoxetine on motor recovery in stroke patients. www.clinicaltrials.gov/ct2/show/NCT02208466 (first received 5 August 2014).
Chen 2019 {published data only}
Choi Kwon 2008 {published data only}
-
- Choi-Kwon S, Choi J, Kwon SU, Kang DW, Kim JS. Fluoxetine improves the quality of life in patients with poststroke emotional disturbances. Cerebrovascular Diseases 2008;26(3):266-71. - PubMed
Finkenzeller 2009 {published data only}
-
- Finkenzeller W, Zobel I, Rietz S, Schramm E, Berger M. Interpersonal psychotherapy and pharmacotherapy for post-stroke depression. Feasibility and effectiveness. Der Nervenarzt 2009;80(7):805-12. - PubMed
Foster 2019 {published data only}
-
- Foster E, Yaroslavtseva O, Chundamala J, Leibovitch F, Eng JJ, Ali F, et al. FLOW Trial: fluoxetine to open the critical period time window to improve motor recovery after stroke. International Journal of Stroke 2019;14 Suppl 3:36.
Gourab 2015 {published data only}
-
- Gourab K, Schmit BD, Hornby GT. Increased lower limb spasticity but not strength or function following a single-dose serotonin reuptake inhibitor in chronic stroke. Archives of Physical Medicine and Rehabilitation 2015;96(12):2112-9. - PubMed
Graffagnino 2002 {published data only}
-
- Graffagnino C. Poststroke depression and functional recovery (SADBRAIN). Duke University Medical Centre 2002.
Ji 2000 {published data only}
-
- Ji QM, Xie LP. Efficacy of fluoxetine in the treatment of 20 patients with depression after stroke. Herald of Medicine 2000;19(4):329.
Kitago 2020 {published data only}
-
- Dose-finding study of selective serotonin reuptake inhibitors to enhance neuroplasticity [Dose finding study but the intervention is a combination of an SSRI and paired associative stimulation, so we can exclude]. Source not provided from searches 2020.
Li 2002 {published data only}
-
- Li F, Gu DX, Ceng SH, Xu JW. Effect of paroxetine on prognosis of patients with post cerebral infarction depression. Chinese Journal of New Drugs and Clinical Remedies. 2002;21(1):11-3.
Liang 2003 {published data only}
-
- Liang Z, Shuliang T. Clinical efficacy of fluoxetine in treatment of patients with depression after acute stroke. Chinese Journal of Clinical Rehabilitation 2003;7(13):1924-5.
Liu 2004 {published data only}
-
- Liu L-X. Recent effect of drug intervention on post-stroke anxiety. Chinese Journal of Clinical Rehabilitation 2004;8(30):6600-1.
Liu 2020 {published data only}
-
- Liu W, Ding W. Study on the efficacy and mechanism of paroxetine hydrochloride combined with repetitive transcranial magnetic stimulation in the treatment of post-stroke depression. International Journal of Clinical and Experimental Medicine 2020;13(10):7881-8.
Mosarrezaii 2018 {published data only}
-
- Mosarrezaii A, Salmasi BA, Taghavi SA. Studying effect of fluoxetine on improvement of motor performance in patients with ischemic stroke. Journal of Research in Medical and Dental Sciences 2018;6(3):118-22.
NCT01963832 {published data only}
-
- NCT01963832. RCT of a neuroplasticity agent and CI therapy for severe arm paresis after stroke. clinicaltrials.gov/ct2/show/NCT01963832 (first received 16 October 2013).
Robinson 2011 {published data only}
-
- Robinson RG, Mikami K, Jang M, Jorge RE. Prevention of anxiety disorder after stroke. Journal of Neuropsychiatry and Clinical Neurosciences 2011;23(2):18.
Sitzer 2002 {published data only}
-
- Sitzer M, Huff W, Steckel R. Prevention of poststroke depression after acute ischemic stroke using the selective serotonin reuptake inhibitor sertraline (PreDis-study). Stroke 2002;33(2):651-2.
Sun 2015 {published data only}
-
- Sun YT, Bao YH, Wang SL, Chu JM, Li LP. Efficacy observation on the treatment of post-stroke depression by acupuncture at the acupoints based on ziwuliuzhu and Prozac. Chinese Acupuncture and Moxibustion 2015;355(2):119-22. - PubMed
Vogel 2020 {published data only}
Xu 2007 {published data only}
-
- Xu B, Zhou WY, Zhang SJ. Observation of effect of Wulung capsule in treating post-stroke depression. Chinese Journal of Integrated Traditional and Western Medicine 2007;27(7):640-2. - PubMed
Zhou 2003 {published data only}
-
- Zhou B. Effects of fluoxetine on neurofunctional recovery of non depressed patients after stroke. Chinese Journal of Clinical Rehabilitation 2003;7(3):374-5.
References to studies awaiting assessment
Guo 2016 {published data only}
-
- Guo Y, He Y, Tang B, Ma K, Cai Z, Zeng S, et al. Effect of using fluoxetine at different time windows on neurological functional prognosis after ischemic stroke. Restorative Neurology and Neuroscience 2016;34(2):177–87. - PubMed
-
- Guo Y. Effect of using fluoxetine at different time windows after ischemic stroke on neurological functional prognosis: a randomized controlled trial. chictr.org.cn/showprojen.aspx?proj=12925 (first received 27 December 2015).
He 2018 {published data only}
-
- ChiCTR-TRC-12002078. Multi-center randomized clinical study of antidepressant treatment (fluoxetine) on secondary prevention of ischemic stroke. www.chictr.org.cn/showprojen.aspx?proj=7471 (first received 3 April 2012).
-
- He Y, Cai Z, Zeng S, Chen S, Tang B, Liang Y, et al. Effect of fluoxetine on three-year recurrence in acute ischemic stroke: a randomized controlled clinical study. Clinical Neurology and Neurosurgery 2018;168:1-6. - PubMed
Jurcau 2016 {published data only}
-
- Jurcau A, Simion A. Improved post-ischaemic stroke recovery over 1 year with escitalopram for 3 months. European Journal of Neurology 2016;23:614.
NCT00967408 {published data only}
-
- NCT00967408. Effects of clinical and functional outcome of escitalopram in adult stroke patients. clinicaltrials.gov/ct2/show/NCT00967408 (first received 27 August 2009). [NCT00967408]
References to ongoing studies
ChiCTR1800019467 {published data only}
-
- ChiCTR1800019467. The effect and mechanism of fluoxetine on the automatic regulation of cerebral blood flow for ischemic stroke. https://www.chictr.org.cn/showprojen.aspx?proj=32801.
CTRI/2018/12/016568 {published data only}
-
- CTRI/2018/12/016568. An interventional study to look at efficacy of fluoxetine in patients with post-stroke anxiety. http://ctri.nic.in CTRI/2018/12/016568 (first received 10 December 2018).
EudraCT 2005‐005266‐37 {published data only}2005‐005266‐37
-
- EudraCT 2005-005266-37. Influence of escitalopram on the incidence of depression and dementia following acute middle cerebral artery territory infarction. A randomised placebo-controlled double blind study. www.clinicaltrialsregister.eu/ctr-search/search?query=2005-005266-37 (first received 7 April 2006).
IRCT201112228490N1 {published data only}
-
- IRCT201112228490N1. Effect of fluoxetine on functional recovery of patients with cerebrovascular accident following middle cerebral artery trunk obstruction: a randomized clinical trial. www.en.irct.ir/trial/8954 (first received 25 January 2012).
IRCT2012101011062N1 {published data only}
-
- IRCT2012101011062N1. A study of sertraline effect on quality of life in stroke inpatients. www.en.irct.ir/trial/11413 (first received 28 November 2012).
IRCT2017041720258N37 {published data only}
-
- IRCT2017041720258N37. Evaluation of fluoxetine and standard treatment efficacy on change to side effect of stroke of ischemic strokes in both hemispheres in anterior circulation. www.irct.ir/trial/17976 (first received 11 October 2017).
IRCT20210307050617N1 {published data only}
-
- IRCT20210307050617N1. The efficacy comparison of fluoxetine and citalopram on motor recovery after ischemic stroke: single-blind placebo-controlled randomized clinical trial. https://en.irct.ir/trial/54896 (first received 27 March 2021).
NCT02386475 {published data only}
-
- NCT02386475. Effect of serotonin and levodopa in ischemic stroke. www.clinicaltrials.gov/ct2/show/NCT02386475 (first received 12 March 2015).
NCT02767999 {published data only}
-
- NCT02767999. Serotonin Selective Reuptake Inhibitor (SSRI) effects on cerebral connectivity in acute ischemic stroke (RECONISE). www.clinicaltrials.gov/ct2/show/NCT02767999 (first received 11 May 2016).
NCT02865642 {published data only}
-
- NCT02865642. Cortical ischemic stroke and serotonin (CISS). www.clinicaltrials.gov/ct2/show/NCT02865642 (first received 12 August 2016).
NCT03448159 {published data only}
-
- NCT03448159. Fluoxetine Opens Window to improve motor recovery after stroke (FLOW). www.clinicaltrials.gov/ct2/show/NCT03448159 (first received 27 February 2018).
NCT03826875 {published data only}
-
- NCT03826875. Depression in hemorrhagic stroke. www.clinicaltrials.gov/ct2/show/NCT03826875 (first received 1 February 2019).
TCTR20181216001 {published data only}
-
- TCTR20181216001. Randomized controlled trial of fluoxetine or placebo on quality of life after acute ischemic stroke. www.thaiclinicaltrials.org/show/TCTR20181216001 (first received 16 December 2018).
Additional references
Allida 2019
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editors. Chapter 10 : Analysing data and performing metaanalyses. In: Higgins JP , Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al( editor s) . ) , Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) Cochrane 2021. Available at www.training.cochrane.org/handbook.
Egger 1997
GBD 2015
-
- GBD 2015. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study. Lancet 2016;388(10053):1545–602. - PMC - PubMed
GRADE 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEproGDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.) GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.), 2015.
Hatano 1976
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Johnson 2016
Lang 2004
-
- Lang UE, Jockers-Scherübl MC, Hellweg R. State of the art of the neurotrophin hypothesis in psychiatric disorders: implications and limitations. Journal of Neural Transmission 2004;111(3):387-411. - PubMed
Legg 2019
Li 2020
-
- Li, X, Zhang C. Comparative efficacy of nine antidepressants in treating Chinese patients with post-stroke depression: a network meta-analysis. Journal of Affective Disorders 2020;266:540-8. - PubMed
Lim 2009
-
- Lim CM, Kim SW, Park JY, Kim C, Yoon SH, Lee JK. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. Journal of Neuroscience Research 2009;87(4):1037-45. - PubMed
Loubinoux 1999
-
- Loubinoux I, Boulanouar K, Ranjeva JP, Carel C, Berry I, Rascol O, et al. Cerebral functional magnetic resonance imaging activation modulated by a single dose of the monoamine neurotransmission enhancers fluoxetine and fenozolone during hand sensorimotor tasks. Journal of Cerebral Blood Flow and Metabolism 1999;19(12):1365-75. - PubMed
Mantel 1959
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719-48. - PubMed
Mead 2020
-
- Mead GE, Graham C, Billot L, Näsman P, Lundström E, Lewis S, for FOCUS, AFFINITY and EFFECTS trialists. Update to the FOCUS, AFFINITY and EFFECTS trials studying the effect(s) of fluoxetine in patients with a recent stroke: statistical analysis plan for the trials and for the individual patient data meta-analysis. Trials 2020;21(1):971. [DOI: ] - PMC - PubMed
Ming 2005
-
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annual Review of Neuroscience 2005;28:223-50. - PubMed
Molnar 2008
NHS 2021
-
- NHS. Side effects-selective serotonin reuptake inhibitors (SSRI). www.nhs.uk (accessed 10 August 2021).
Pälvimäki 1994
-
- Pälvimäki E-P, Laakso A, Kuoppamäki M, Syvälahti E, Hietala J. Up-regulation of beta l-adrenergic receptors in rat brain after chronic citalopram and fluoxetine treatments. Psychopharmacology 1994;115(4):543-6. - PubMed
Pinto 2017
Review Manager 2014 [Computer program]
-
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Santarelli 2003
-
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis antidepressant treatments and animal models of depressive-like behaviour. Behavioural Pharmacology 2003;301:805-9. - PubMed
Schmidt 2007
-
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behaviour. Behavioural Pharmacology 2007;18(5-6):391-418. - PubMed
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, Guyatt GH on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Shin 2009
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Taupin 2006
-
- Taupin P. Adult neurogenesis and neuroplasticity. Restorative Neurology and Neuroscience 2006;24(1):9-15. - PubMed
Wan 2014
Wiltrout 2007
-
- Wiltrout C, Lang B, Yan Y, Dempsey RJ, Vemuganti R. Repairing brain after stroke: a review on post-ischaemic neurogenesis. Neurochemistry International 2007;50(7-8):1028-41. - PubMed
Xie 2019
-
- Xie Y, Liu PP, Lian YJ, Liu H-B, Kang J-S. The effect of selective serotonin reuptake inhibitors on cognitive function in patients with Alzheimer’s disease and vascular dementia: focusing on fluoxetine with long follow-up periods. Signal Transduction and Targeted Therapy 2019;4:30. [DOI: 10.1038/s41392-019-0064-7] - DOI - PMC - PubMed
Yi 2010
-
- Yi ZM, Liu F, Zhai SD. Fluoxetine for the prophylaxis of post-stroke depression in patients with stroke: a meta-analysis. International Journal of Clinical Practice 2010;64(9):1310-7. - PubMed
References to other published versions of this review
Legg 22019
Mead 2011
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

