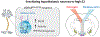Running the Female Power Grid Across Lifespan Through Brain Estrogen Signaling
- PMID: 34780257
- PMCID: PMC8831472
- DOI: 10.1146/annurev-physiol-061121-035914
Running the Female Power Grid Across Lifespan Through Brain Estrogen Signaling
Abstract
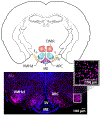
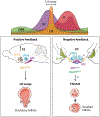
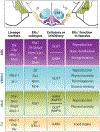
The role of central estrogen in cognitive, metabolic, and reproductive health has long fascinated the lay public and scientists alike. In the last two decades, insight into estrogen signaling in the brain and its impact on female physiology is beginning to catch up with the vast information already established for its actions on peripheral tissues. Using newer methods to manipulate estrogen signaling in hormone-sensitive brain regions, neuroscientists are now identifying the molecular pathways and neuronal subtypes required for controlling sex-dependent energy allocation. However, the immense cellular complexity of these hormone-sensitive brain regions makes it clear that more research is needed to fully appreciate how estrogen modulates neural circuits to regulate physiological and behavioral end points. Such insight is essential for understanding how natural or drug-induced hormone fluctuations across lifespan affect women's health.
Keywords: arcuate nucleus; brain-bone connection; central estrogen signaling; estrogen receptor alpha; female physiology; hypothalamus; menopause; reproduction; sex-dependent neurocircuits; ventromedial hypothalamus; women's health.
Figures