Milvexian for the Prevention of Venous Thromboembolism
- PMID: 34780683
- PMCID: PMC9540352
- DOI: 10.1056/NEJMoa2113194
Milvexian for the Prevention of Venous Thromboembolism
Abstract
Background: Factor XIa inhibitors for the prevention and treatment of venous and arterial thromboembolism may be more effective and result in less bleeding than conventional anticoagulants. Additional data are needed regarding the efficacy and safety of milvexian, an oral factor XIa inhibitor.
Methods: In this parallel-group, phase 2 trial, we randomly assigned 1242 patients undergoing knee arthroplasty to receive one of seven postoperative regimens of milvexian (25 mg, 50 mg, 100 mg, or 200 mg twice daily or 25 mg, 50 mg, or 200 mg once daily) or enoxaparin (40 mg once daily). The primary efficacy outcome was venous thromboembolism (which was a composite of asymptomatic deep-vein thrombosis, confirmed symptomatic venous thromboembolism, or death from any cause). The principal safety outcome was bleeding.
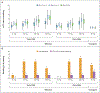
Results: Among the patients receiving milvexian twice daily, venous thromboembolism developed in 27 of 129 (21%) taking 25 mg, in 14 of 124 (11%) taking 50 mg, in 12 of 134 (9%) taking 100 mg, and in 10 of 131 (8%) taking 200 mg. Among those receiving milvexian once daily, venous thromboembolism developed in 7 of 28 (25%) taking 25 mg, in 30 of 127 (24%) taking 50 mg, and in 8 of 123 (7%) taking 200 mg, as compared with 54 of 252 patients (21%) taking enoxaparin. The dose-response relationship with twice-daily milvexian was significant (one-sided P<0.001), and the 12% incidence of venous thromboembolism with twice-daily milvexian was significantly lower than the prespecified benchmark of 30% (one-sided P<0.001). Bleeding of any severity occurred in 38 of 923 patients (4%) taking milvexian and in 12 of 296 patients (4%) taking enoxaparin; major or clinically relevant nonmajor bleeding occurred in 1% and 2%, respectively; and serious adverse events were reported in 2% and 4%, respectively.
Conclusions: Postoperative factor XIa inhibition with oral milvexian in patients undergoing knee arthroplasty was effective for the prevention of venous thromboembolism and was associated with a low risk of bleeding. (Funded by Bristol Myers Squibb and Janssen Research and Development; AXIOMATIC-TKR ClinicalTrials.gov number, NCT03891524.).
Copyright © 2021 Massachusetts Medical Society.
Figures

Comment in
-
Inhibiting factor XIa to prevent thromboembolism.Nat Rev Cardiol. 2022 Feb;19(2):78. doi: 10.1038/s41569-021-00660-y. Nat Rev Cardiol. 2022. PMID: 34824457 No abstract available.
-
Can targeting factor XIa dissociate thrombosis from haemostasis?Eur Heart J. 2022 Mar 14;43(11):1031-1032. doi: 10.1093/eurheartj/ehab871. Eur Heart J. 2022. PMID: 34974621 No abstract available.
References
-
- Steinberg BA, Gao H, Shrader P, et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J 2017; 194: 132–40. - PubMed
-
- Duga S, Salomon O. Congenital factor XI deficiency: an update. Semin Thromb Hemost 2013; 39: 621–31. - PubMed
-
- Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost 2011; 105: 269–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
