Total Biosynthesis of the Tubulin-Binding Alkaloid Colchicine
- PMID: 34780686
- PMCID: PMC10824269
- DOI: 10.1021/jacs.1c08659
Total Biosynthesis of the Tubulin-Binding Alkaloid Colchicine
Abstract
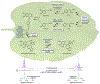
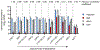
Colchicine (1) is a bioactive plant alkaloid from Colchicum and Gloriosa species that is used as a pharmaceutical treatment for inflammatory diseases, including gouty arthritis and familial Mediterranean fever. The activity of this alkaloid is attributed to its ability to bind tubulin dimers and inhibit microtubule assembly, which not only promotes anti-inflammatory effects, but also makes colchicine a potent mitotic poison. The biochemical origins of colchicine biosynthesis have been investigated for over 50 years, but only recently has the underlying enzymatic machinery become clear. Here, we report the discovery of multiple pathway enzymes from Gloriosa superba that allows for the reconstitution of a complete metabolic route to 1. This includes three enzymes that process a previously established tropolone-containing intermediate into 1 via tailoring of the nitrogen atom. We further demonstrate the total biosynthesis of enantiopure (-)-1 from primary metabolites via heterologous production in a model plant, thus enabling future efforts for the metabolic engineering of this medicinal alkaloid. Additionally, our results provide insight into the timing and tissue specificity for the late stage modifications required in colchicine biosynthesis, which are likely connected to the biological functions for this class of medicinal alkaloids in native producing plants.
Figures

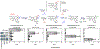
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

