Preclinical investigation of Pegylated arginase 1 as a treatment for retina and brain injury
- PMID: 34780773
- PMCID: PMC9122100
- DOI: 10.1016/j.expneurol.2021.113923
Preclinical investigation of Pegylated arginase 1 as a treatment for retina and brain injury
Abstract
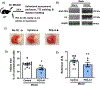
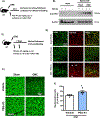
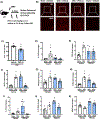
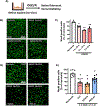
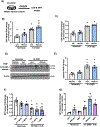
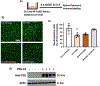
Arginase 1 (A1) is the enzyme that hydrolyzes the amino acid, L-arginine, to ornithine and urea. We have previously shown that A1 deletion worsens retinal ischemic injury, suggesting a protective role of A1. In this translational study, we aimed to study the utility of systemic pegylated A1 (PEG-A1, recombinant human arginase linked to polyethylene glycol) treatment in mouse models of acute retinal and brain injury. Cohorts of WT mice were subjected to retinal ischemia-reperfusion (IR) injury, traumatic optic neuropathy (TON) or brain cerebral ischemia via middle cerebral artery occlusion (MCAO) and treated with intraperitoneal injections of PEG-A1 or vehicle (PEG only). Drug penetration into retina and brain tissues was measured by western blotting and immunolabeling for PEG. Neuroprotection was measured in a blinded fashion by quantitation of NeuN (neuronal marker) immunolabeling of retina flat-mounts and brain infarct area using triphenyl tetrazolium chloride (TTC) staining. Furthermore, ex vivo retina explants and in vitro retina neuron cultures were subjected to oxygen-glucose deprivation (OGD) followed by reoxygenation (R) and treated with PEG-A1. PEG-A1 given systemically did not cross the intact blood-retina/brain barriers in sham controls but reached the retina and brain after injury. PEG-A1 provided neuroprotection after retinal IR injury, TON and cerebral ischemia. PEG-A1 treatment was also neuroprotective in retina explants subjected to OGD/R but did not improve survival in retinal neuronal cultures exposed to OGD/R. In summary, systemic PEG-A1 administration is neuroprotective and provides an excellent route to deliver the drug to the retina and the brain after acute injury.
Keywords: Arginase; Ischemia-reperfusion injury; Neurodegeneration; Neuroprotection; Retinal ischemia; Stroke.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
AF, RBC, and RWC have a pending patent on the use of arginase 1 as a treatment for ischemic retinopathies. PNMC is the chief executive officer of Bio-Cancer Treatment International Limited and holds stocks or shares in Bio-Cancer Treatment International Limited. The other authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

