SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein
- PMID: 34780863
- PMCID: PMC8590447
- DOI: 10.1016/j.nbd.2021.105561
SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein
Abstract
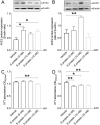
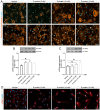
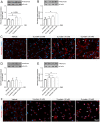
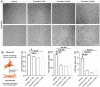
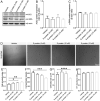
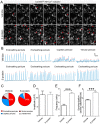
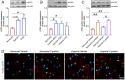
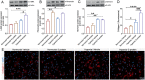
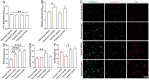
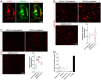
Coronavirus disease 19 (COVID-19) is a respiratory illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). COVID-19 pathogenesis causes vascular-mediated neurological disorders via elusive mechanisms. SARS-CoV-2 infects host cells via the binding of viral Spike (S) protein to transmembrane receptor, angiotensin-converting enzyme 2 (ACE2). Although brain pericytes were recently shown to abundantly express ACE2 at the neurovascular interface, their response to SARS-CoV-2 S protein is still to be elucidated. Using cell-based assays, we found that ACE2 expression in human brain vascular pericytes was increased upon S protein exposure. Pericytes exposed to S protein underwent profound phenotypic changes associated with an elongated and contracted morphology accompanied with an enhanced expression of contractile and myofibrogenic proteins, such as α-smooth muscle actin (α-SMA), fibronectin, collagen I, and neurogenic locus notch homolog protein-3 (NOTCH3). On the functional level, S protein exposure promoted the acquisition of calcium (Ca2+) signature of contractile ensheathing pericytes characterized by highly regular oscillatory Ca2+ fluctuations. Furthermore, S protein induced lipid peroxidation, oxidative and nitrosative stress in pericytes as well as triggered an immune reaction translated by activation of nuclear factor-kappa-B (NF-κB) signaling pathway, which was potentiated by hypoxia, a condition associated with vascular comorbidities that exacerbate COVID-19 pathogenesis. S protein exposure combined to hypoxia enhanced the production of pro-inflammatory cytokines involved in immune cell activation and trafficking, namely macrophage migration inhibitory factor (MIF). Using transgenic mice expressing the human ACE2 that recognizes S protein, we observed that the intranasal infection with SARS-CoV-2 rapidly induced hypoxic/ischemic-like pericyte reactivity in the brain of transgenic mice, accompanied with an increased vascular expression of ACE2. Moreover, we found that SARS-CoV-2 S protein accumulated in the intranasal cavity reached the brain of mice in which the nasal mucosa is deregulated. Collectively, these findings suggest that SARS-CoV-2 S protein impairs the vascular and immune regulatory functions of brain pericytes, which may account for vascular-mediated brain damage. Our study provides a better understanding for the mechanisms underlying cerebrovascular disorders in COVID-19, paving the way to develop new therapeutic interventions.
Keywords: COVID-19; Cerebrovascular disorders; Inflammation; Myofibrogenic transition; Neurovascular interface; Pericytes; SARS-CoV-2 S protein.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Alisson-Silva F., Freire-de-Lima L., Donadio J.L., Lucena M.C., Penha L., Sá-Diniz J.N., et al. Increase of O-glycosylated oncofetal fibronectin in high glucose-induced epithelial-mesenchymal transition of cultured human epithelial cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060471. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

