Interleukin-6 in SARS-CoV-2 induced disease: Interactions and therapeutic applications
- PMID: 34781146
- PMCID: PMC8585600
- DOI: 10.1016/j.biopha.2021.112419
Interleukin-6 in SARS-CoV-2 induced disease: Interactions and therapeutic applications
Abstract
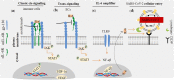
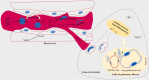
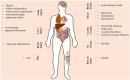
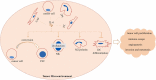
Interleukin-6 (IL-6) is a multi-tasking cytokine that represents high activity in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and cancer. High concentration of this pleiotropic cytokine accounts for hyperinflammation and cytokine storm, and is related to multi-organ failure in patients with SARS-CoV-2 induced disease. IL-6 promotes lymphopenia and increases C-reactive protein (CRP) in such cases. However, blockade of IL-6 is not a full-proof of complete response. Hypoxia, hypoxemia, aberrant angiogenesis and chronic inflammation are inter-related events occurring as a response to the SARS-CoV-2 stimulatory effect on high IL-6 activity. Taking both pro- and anti-inflammatory activities will make complex targeting IL-6 in patient with SARS-CoV-2 induced disease. The aim of this review was to discuss about interactions occurring within the body of patients with SARS-CoV-2 induced disease who are representing high IL-6 levels, and to determine whether IL-6 inhibition therapy is effective for such patients or not. We also address the interactions and targeted therapies in cancer patients who also have SARS-CoV-2 induced disease.
Keywords: C-reactive protein (CRP); Cancer; Cytokine storm; Hypoxia; Inflammation; Interleukin-6 (IL-6); Pneumonia; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); Tocilizumab.
Copyright © 2021 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
None.
Figures
Similar articles
-
Therapeutic Role of Tocilizumab in SARS-CoV-2-Induced Cytokine Storm: Rationale and Current Evidence.Int J Mol Sci. 2021 Mar 17;22(6):3059. doi: 10.3390/ijms22063059. Int J Mol Sci. 2021. PMID: 33802761 Free PMC article. Review.
-
Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 3;21(1):468. doi: 10.1186/s13063-020-04453-5. Trials. 2020. PMID: 32493441 Free PMC article.
-
Tocilizumab in SARS-CoV-2 Patients with the Syndrome of Cytokine Storm: A Narrative Review.Rev Recent Clin Trials. 2021;16(2):138-145. doi: 10.2174/1574887115666200917110954. Rev Recent Clin Trials. 2021. PMID: 32940187 Review.
-
Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial.Lancet Respir Med. 2021 Dec;9(12):1427-1438. doi: 10.1016/S2213-2600(21)00377-5. Epub 2021 Oct 29. Lancet Respir Med. 2021. PMID: 34756178 Free PMC article. Clinical Trial.
-
Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review.J Med Virol. 2020 Nov;92(11):2516-2522. doi: 10.1002/jmv.26038. Epub 2020 Jun 9. J Med Virol. 2020. PMID: 32436994 Free PMC article.
Cited by
-
IL-6 and IL-17 as potential links between pre-existing hypertension and long-term COVID sequelae in patients undergoing hemodialysis: a multicenter cross-sectional study.Sci Rep. 2024 Feb 29;14(1):4968. doi: 10.1038/s41598-024-54930-z. Sci Rep. 2024. PMID: 38424126 Free PMC article.
-
Checkpoint inhibitor/interleukin-based combination therapy of cancer.Cancer Med. 2022 Aug;11(15):2934-2943. doi: 10.1002/cam4.4659. Epub 2022 Mar 17. Cancer Med. 2022. PMID: 35301813 Free PMC article. Review.
-
Ongoing Use of SSRIs Does Not Alter Outcome in Hospitalized COVID-19 Patients: A Retrospective Analysis.J Clin Med. 2021 Dec 24;11(1):70. doi: 10.3390/jcm11010070. J Clin Med. 2021. PMID: 35011811 Free PMC article.
-
Effects of Child-Friendly Music Nursing in the Ward on Mental Health of Children with Henoch-Schönlein Purpura Nephritis: A Retrospective Study.Noise Health. 2025 Jan-Feb 01;27(124):20-25. doi: 10.4103/nah.nah_127_24. Epub 2025 Feb 28. Noise Health. 2025. PMID: 40029674 Free PMC article.
-
Epithelial-mesenchymal transition in cancer stemness and heterogeneity: updated.Med Oncol. 2022 Sep 7;39(12):193. doi: 10.1007/s12032-022-01801-0. Med Oncol. 2022. PMID: 36071302 Review.
References
-
- Lescure F.-X., Honda H., Fowler R.A., Lazar J.S., Shi G., Wung P., Patel N., Hagino O., Bazzalo I.J., Casas M.M. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021;9(5):522–532. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

