Azacitidine is a potential therapeutic drug for pyridoxine-refractory female X-linked sideroblastic anemia
- PMID: 34781359
- PMCID: PMC8864662
- DOI: 10.1182/bloodadvances.2021005664
Azacitidine is a potential therapeutic drug for pyridoxine-refractory female X-linked sideroblastic anemia
Abstract
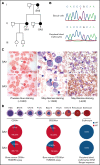
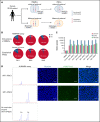
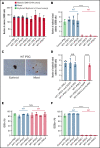
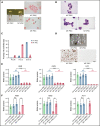
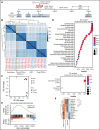
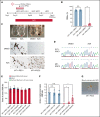
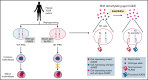
X-linked sideroblastic anemia (XLSA) is associated with mutations in the erythroid-specific δ-aminolevulinic acid synthase (ALAS2) gene. Treatment of XLSA is mainly supportive, except in patients who are pyridoxine responsive. Female XLSA often represents a late onset of severe anemia, mostly related to the acquired skewing of X chromosome inactivation. In this study, we successfully generated active wild-type and mutant ALAS2-induced pluripotent stem cell (iPSC) lines from the peripheral blood cells of an affected mother and 2 daughters in a family with pyridoxine-resistant XLSA related to a heterozygous ALAS2 missense mutation (R227C). The erythroid differentiation potential was severely impaired in active mutant iPSC lines compared with that in active wild-type iPSC lines. Most of the active mutant iPSC-derived erythroblasts revealed an immature morphological phenotype, and some showed dysplasia and perinuclear iron deposits. In addition, globin and HO-1 expression and heme biosynthesis in active mutant erythroblasts were severely impaired compared with that in active wild-type erythroblasts. Furthermore, genes associated with erythroblast maturation and karyopyknosis showed significantly reduced expression in active mutant erythroblasts, recapitulating the maturation defects. Notably, the erythroid differentiation ability and hemoglobin expression of active mutant iPSC-derived hematopoietic progenitor cells (HPCs) were improved by the administration of δ-aminolevulinic acid, verifying the suitability of the cells for drug testing. Administration of a DNA demethylating agent, azacitidine, reactivated the silent, wild-type ALAS2 allele in active mutant HPCs and ameliorated the erythroid differentiation defects, suggesting that azacitidine is a potential novel therapeutic drug for female XLSA. Our patient-specific iPSC platform provides novel biological and therapeutic insights for XLSA.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

Similar articles
-
Late-onset X-linked sideroblastic anemia. Missense mutations in the erythroid delta-aminolevulinate synthase (ALAS2) gene in two pyridoxine-responsive patients initially diagnosed with acquired refractory anemia and ringed sideroblasts.J Clin Invest. 1995 Oct;96(4):2090-6. doi: 10.1172/JCI118258. J Clin Invest. 1995. PMID: 7560104 Free PMC article.
-
The role of genetic testing in accurate diagnosis of X-linked sideroblastic anemia: novel ALAS2 mutations and the impact of X-chromosome inactivation.Sci Rep. 2025 Apr 7;15(1):11843. doi: 10.1038/s41598-025-95590-x. Sci Rep. 2025. PMID: 40195342 Free PMC article.
-
Familial-skewed X-chromosome inactivation as a predisposing factor for late-onset X-linked sideroblastic anemia in carrier females.Blood. 2000 Dec 15;96(13):4363-5. Blood. 2000. PMID: 11110715
-
Regulation and tissue-specific expression of δ-aminolevulinic acid synthases in non-syndromic sideroblastic anemias and porphyrias.Mol Genet Metab. 2019 Nov;128(3):190-197. doi: 10.1016/j.ymgme.2019.01.015. Epub 2019 Jan 23. Mol Genet Metab. 2019. PMID: 30737140 Review.
-
Animal models for X-linked sideroblastic anemia.Int J Hematol. 2000 Aug;72(2):157-64. Int J Hematol. 2000. PMID: 11039663 Review.
Cited by
-
Pulling back the mitochondria's iron curtain.NPJ Metab Health Dis. 2025;3(1):6. doi: 10.1038/s44324-024-00045-y. Epub 2025 Mar 4. NPJ Metab Health Dis. 2025. PMID: 40052109 Free PMC article. Review.
-
An erythroid-specific lentiviral vector improves anemia and iron metabolism in a new model of XLSA.Blood. 2025 Jan 2;145(1):98-113. doi: 10.1182/blood.2024025846. Blood. 2025. PMID: 39656107
-
Murine models of erythroid 5ALA synthesis disorders and their conditional synthetic lethal dependency on pyridoxine.Blood. 2024 Sep 26;144(13):1418-1432. doi: 10.1182/blood.2023023078. Blood. 2024. PMID: 38900972
-
Modeling Reduced Contractility and Stiffness Using iPSC-Derived Cardiomyocytes Generated From Female Becker Muscular Dystrophy Carrier.JACC Basic Transl Sci. 2023 Feb 8;8(6):599-613. doi: 10.1016/j.jacbts.2022.11.007. eCollection 2023 Jun. JACC Basic Transl Sci. 2023. PMID: 37426526 Free PMC article.
-
Three-generation female cohort with macrocytic anemia and iron overload.Am J Hematol. 2025 Jan;100(1):133-138. doi: 10.1002/ajh.27489. Epub 2024 Sep 27. Am J Hematol. 2025. PMID: 39329459 Free PMC article. No abstract available.
References
-
- Bottomley SS, Fleming MD. Sideroblastic anemia: diagnosis and management. Hematol Oncol Clin North Am. 2014;28(4):653-670, v. - PubMed
-
- Aivado M, Gattermann N, Rong A, et al. . X-linked sideroblastic anemia associated with a novel ALAS2 mutation and unfortunate skewed X-chromosome inactivation patterns. Blood Cells Mol Dis. 2006;37(1):40-45. - PubMed

