Wnt-β-Catenin Signaling in Human Dendritic Cells Mediates Regulatory T-Cell Responses to Fungi via the PD-L1 Pathway
- PMID: 34781737
- PMCID: PMC8593687
- DOI: 10.1128/mBio.02824-21
Wnt-β-Catenin Signaling in Human Dendritic Cells Mediates Regulatory T-Cell Responses to Fungi via the PD-L1 Pathway
Abstract
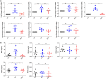
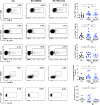
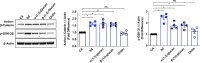
The signaling pathways activated following interaction between dendritic cells (DCs) and a pathogen determine the polarization of effector T-cell and regulatory T-cell (Treg) responses to the infection. Several recent studies, mostly in the context of bacterial infections, have shown that the Wnt/β-catenin pathway plays a major role in imparting tolerogenic features in DCs and in promotion of Treg responses. However, the significance of the Wnt/β-catenin pathway's involvement in regulating the immune response to the fungal species is not known. Using Aspergillus fumigatus, a ubiquitous airborne opportunistic fungal species, we show here that fungi activate the Wnt/β-catenin pathway in human DCs and are critical for mediating the immunosuppressive Treg responses. Pharmacological inhibition of this pathway in DCs led to inhibition of maturation-associated molecules and interleukin 10 (IL-10) secretion without affecting the majority of the inflammatory cytokines. Furthermore, blockade of Wnt signaling in DCs suppressed DC-mediated Treg responses in CD4+ T cells and downregulated both tumor necrosis factor alpha (TNF-α) and IL-10 responses in CD8+ T cells. Mechanistically, induction of β-catenin pathway by A. fumigatus required C-type lectin receptors and promoted Treg polarization via the induction of programmed death-ligand 1 on DCs. Further investigation on the identity of fungal molecular patterns has revealed that the cell wall polysaccharides β-(1, 3)-glucan and α-(1, 3)-glucan, but not chitin, possess the capacity to activate the β-catenin pathway. Our data suggest that the Wnt/β-catenin pathway is a potential therapeutic target to selectively suppress the Treg response and to sustain the protective Th1 response in the context of invasive aspergillosis caused by A. fumigatus. IMPORTANCE The balance between effector CD4+ T-cell and immunosuppressive regulatory T-cell (Treg) responses determines the outcome of an infectious disease. The signaling pathways that regulate human CD4+ T-effector versus Treg responses to the fungi are not completely understood. By using Aspergillus fumigatus, a ubiquitous opportunistic fungal species, we show that fungi activate the Wnt/β-catenin pathway in human dendritic cells (DCs) that promotes Treg responses via induction of immune checkpoint molecule programmed death ligand 1 on DCs. Blockade of the Wnt/β-catenin pathway in DCs led to the selective inhibition of Treg without affecting the Th1 response. Dissection of the identity of A. fumigatus pathogen-associated molecular patterns (PAMPs) revealed that cell wall polysaccharides exhibit selectivity in their capacity to activate the β-catenin pathway in DCs. Our data thus provide a pointer that Wnt/β-catenin pathway represents potential therapeutic target to selectively suppress Treg responses and to sustain protective a Th1 response against invasive fungal diseases.
Keywords: Aspergillus fumigatus; DC-SIGN; PD-L1; Wnt signaling; Wnt-β-catenin; dectin-1; dectin-2; dendritic cells; human; regulatory T cells.
Figures





Similar articles
-
Aspergillus fumigatus Cell Wall α-(1,3)-Glucan Stimulates Regulatory T-Cell Polarization by Inducing PD-L1 Expression on Human Dendritic Cells.J Infect Dis. 2017 Dec 5;216(10):1281-1294. doi: 10.1093/infdis/jix469. J Infect Dis. 2017. PMID: 28968869
-
Programmed death ligand 1 (PD-L1) plays a vital part in DC tolerogenicity induced by IFN-γ.Int Immunopharmacol. 2021 Oct;99:107978. doi: 10.1016/j.intimp.2021.107978. Epub 2021 Jul 20. Int Immunopharmacol. 2021. PMID: 34298399
-
Dendritic Cells Exposed to Triiodothyronine Deliver Pro-Inflammatory Signals and Amplify IL-17-Driven Immune Responses.Cell Physiol Biochem. 2019;52(2):354-367. doi: 10.33594/000000025. Epub 2019 Feb 28. Cell Physiol Biochem. 2019. PMID: 30816679
-
Targeting Wnt/β-Catenin Signaling for Cancer Immunotherapy.Trends Pharmacol Sci. 2018 Jul;39(7):648-658. doi: 10.1016/j.tips.2018.03.008. Epub 2018 Apr 17. Trends Pharmacol Sci. 2018. PMID: 29678298 Review.
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
Cited by
-
Fungi and cancer: unveiling the complex role of fungal infections in tumor biology and therapeutic resistance.Front Cell Infect Microbiol. 2025 Jun 10;15:1596688. doi: 10.3389/fcimb.2025.1596688. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40557321 Free PMC article. Review.
-
Antifungal immunity mediated by C-type lectin receptors may be a novel target in immunotherapy for urothelial bladder cancer.Front Immunol. 2022 Sep 5;13:911325. doi: 10.3389/fimmu.2022.911325. eCollection 2022. Front Immunol. 2022. PMID: 36131933 Free PMC article.
-
The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: a crosstalk between ancient "Yin-Yang" theory and modern immunology.Cell Commun Signal. 2024 Feb 6;22(1):99. doi: 10.1186/s12964-024-01505-0. Cell Commun Signal. 2024. PMID: 38317142 Free PMC article. Review.
-
Retrospective analysis on the immunopotentiating mechanism of an emulsion-based vaccine adjuvant on human antigen presenting cells.Front Immunol. 2023 Jan 9;13:1086752. doi: 10.3389/fimmu.2022.1086752. eCollection 2022. Front Immunol. 2023. PMID: 36700217 Free PMC article.
-
Matrix metalloproteinase-8 regulates dendritic cell tolerance in late polymicrobial sepsis via the nuclear factor kappa-B p65/β-catenin pathway.Burns Trauma. 2024 Feb 28;12:tkad025. doi: 10.1093/burnst/tkad025. eCollection 2024. Burns Trauma. 2024. PMID: 38425412 Free PMC article.
References
-
- Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 50:1101–1111. doi:10.1086/651262. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

