Unexpected Mechanism of Biodegradation and Defluorination of 2,2-Difluoro-1,3-Benzodioxole by Pseudomonas putida F1
- PMID: 34781746
- PMCID: PMC8593668
- DOI: 10.1128/mBio.03001-21
Unexpected Mechanism of Biodegradation and Defluorination of 2,2-Difluoro-1,3-Benzodioxole by Pseudomonas putida F1
Abstract
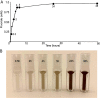
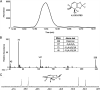
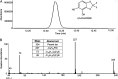
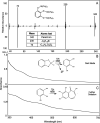
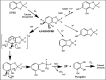
Perfluorinated carbon atoms in a diether linkage are common in commercial anesthetics, drugs, fungicides, and insecticides. An important chemical group comprising perfluorodiethers is the 2,2-fluoro-1,3-benzodioxole (DFBD) moiety. The fluorine atoms stabilize the molecule by mitigating against metabolism by humans and microbes, as used in drugs and pesticides, respectively. Pseudomonas putida F1 catalyzed defluorination of DFBD at an initial rate of 2,100 nmol/h per mg cellular protein. This is orders of magnitude higher than previously reported microbial defluorination rates with multiply fluorinated carbon atoms. Defluorination rates declined after several hours, and the medium darkened. Significant defluorination activity was observed with cells grown on toluene but not l-arginine. Defluorination required only toluene dioxygenase. Pseudomonas and recombinant Escherichia coli cells expressing toluene dioxygenase oxidized DFBD to DFBD-4,5-dihydrodiol. The dihydrodiol could be oxidized to 4,5-dihydroxy-DFBD via the dihydrodiol dehydrogenase from P. putida F1. The dihydrodiol dehydrated with acid to yield a mixture of 4-hydroxy-DFBD and 5-hydroxy-DFBD. All those metabolites retained the difluoromethylene group; no fluoride or dark color was observed. The major route of DFBD-4,5-dihydrodiol decomposition produced fluoride and 1,2,3-trihydroxybenzene, or pyrogallol, and that was shown to be the source of the dark colors in the medium. A mechanism for DFBD-4,5-dihydrodiol transformation to two fluoride ions and pyrogallol is proposed. The Pseudomonas genome database and other databases revealed hundreds of bacteria with enzymes sharing high amino acid sequence identity to toluene dioxygenase from P. putida F1, suggesting the mechanism revealed here may apply to the defluorination of DFBD-containing compounds in the environment. IMPORTANCE There are more than 9,000 polyfluorinated compounds developed for commercial use, some negatively impacting human health, and they are generally considered to be resistant to biodegradation. Only a limited number of studies have identified microbes with enzymes sufficiently reactive to defluorinate difluoromethylene carbon groups. The present study examined one important group of commercial fluorinated chemicals and showed its rapid defluorination by a bacterium and its key enzyme, a Rieske dioxygenase. Rieske dioxygenases are common in environmental bacteria, and those closely resembling toluene dioxygenase from Pseudomonas putida F1 are candidates for biodegradative defluorination of the common 2,2-fluoro-1,3-benzodioxole (DFBD) moiety.
Keywords: PFAS; Pseudomonas putida F1; bacteria; defluorination; dioxygenase; fluoride; organofluorine; oxygenase; pesticides; pyrogallol; rapid rate.
Figures

Similar articles
-
Microwell Fluoride Screen for Chemical, Enzymatic, and Cellular Reactions Reveals Latent Microbial Defluorination Capacity for -CF3 Groups.Appl Environ Microbiol. 2022 May 10;88(9):e0028822. doi: 10.1128/aem.00288-22. Epub 2022 Apr 18. Appl Environ Microbiol. 2022. PMID: 35435713 Free PMC article.
-
Fluoro-recognition: New in vivo fluorescent assay for toluene dioxygenase probing induction by and metabolism of polyfluorinated compounds.Environ Microbiol. 2022 Nov;24(11):5202-5216. doi: 10.1111/1462-2920.16187. Epub 2022 Oct 17. Environ Microbiol. 2022. PMID: 36054238 Free PMC article.
-
Evaluation of aromatic hydrocarbon decomposition catalyzed by the dioxygenase system and substitution of ferredoxin and ferredoxin reductase.Environ Sci Pollut Res Int. 2019 Nov;26(33):34047-34057. doi: 10.1007/s11356-018-3200-y. Epub 2018 Sep 23. Environ Sci Pollut Res Int. 2019. PMID: 30244447
-
The link between ancient microbial fluoride resistance mechanisms and bioengineering organofluorine degradation or synthesis.Nat Commun. 2024 May 30;15(1):4593. doi: 10.1038/s41467-024-49018-1. Nat Commun. 2024. PMID: 38816380 Free PMC article. Review.
-
Confronting PFAS persistence: enzymes catalyzing C-F bond cleavage.Trends Biochem Sci. 2025 Jan;50(1):71-83. doi: 10.1016/j.tibs.2024.11.001. Epub 2024 Dec 5. Trends Biochem Sci. 2025. PMID: 39643519 Review.
Cited by
-
Role of the CrcB transporter of Pseudomonas putida in the multi-level stress response elicited by mineral fluoride.Environ Microbiol. 2022 Nov;24(11):5082-5104. doi: 10.1111/1462-2920.16110. Epub 2022 Jul 1. Environ Microbiol. 2022. PMID: 35726888 Free PMC article.
-
Contrasting Mechanisms of Aromatic and Aryl-Methyl Substituent Hydroxylation by the Rieske Monooxygenase Salicylate 5-Hydroxylase.Biochemistry. 2023 Jan 17;62(2):507-523. doi: 10.1021/acs.biochem.2c00610. Epub 2022 Dec 30. Biochemistry. 2023. PMID: 36583545 Free PMC article.
-
Microwell Fluoride Screen for Chemical, Enzymatic, and Cellular Reactions Reveals Latent Microbial Defluorination Capacity for -CF3 Groups.Appl Environ Microbiol. 2022 May 10;88(9):e0028822. doi: 10.1128/aem.00288-22. Epub 2022 Apr 18. Appl Environ Microbiol. 2022. PMID: 35435713 Free PMC article.
-
Fluoro-recognition: New in vivo fluorescent assay for toluene dioxygenase probing induction by and metabolism of polyfluorinated compounds.Environ Microbiol. 2022 Nov;24(11):5202-5216. doi: 10.1111/1462-2920.16187. Epub 2022 Oct 17. Environ Microbiol. 2022. PMID: 36054238 Free PMC article.
-
Polymeric Amorphous Solid Dispersions of Dasatinib: Formulation and Ecotoxicological Assessment.Pharmaceutics. 2024 Apr 18;16(4):551. doi: 10.3390/pharmaceutics16040551. Pharmaceutics. 2024. PMID: 38675212 Free PMC article.
References
-
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29:131–147. doi:10.1038/s41370-018-0094-1. - DOI - PMC - PubMed
-
- Key BD, Howell RD, Criddle CS. 1997. Fluorinated organics in the biosphere. Environ Sci Technol 31:2445–2454. doi:10.1021/es961007c. - DOI
-
- Smart B. 2001. Fluorine substituent effects (on bioactivity). J Fluor Chem 109:3–11. doi:10.1016/S0022-1139(01)00375-X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

