The emergence of ecotypes in a parasitoid wasp: a case of incipient sympatric speciation in Hymenoptera?
- PMID: 34781897
- PMCID: PMC8591844
- DOI: 10.1186/s12862-021-01938-y
The emergence of ecotypes in a parasitoid wasp: a case of incipient sympatric speciation in Hymenoptera?
Abstract
Background: To understand which reproductive barriers initiate speciation is a major question in evolutionary research. Despite their high species numbers and specific biology, there are only few studies on speciation in Hymenoptera. This study aims to identify very early reproductive barriers in a local, sympatric population of Nasonia vitripennis (Walker 1836), a hymenopterous parasitoid of fly pupae. We studied ecological barriers, sexual barriers, and the reduction in F1-female offspring as a postmating barrier, as well as the population structure using microsatellites.
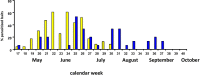
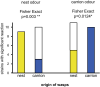
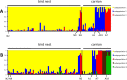
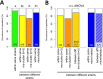
Results: We found considerable inbreeding within female strains and a population structure with either three or five subpopulation clusters defined by microsatellites. In addition, there are two ecotypes, one parasitizing fly pupae in bird nests and the other on carrion. The nest ecotype is mainly formed from one of the microsatellite clusters, the two or four remaining microsatellite clusters form the carrion ecotype. There was slight sexual isolation and a reduction in F1-female offspring between inbreeding strains from the same microsatellite clusters and the same ecotypes. Strains from different microsatellite clusters are separated by a reduction in F1-female offspring. Ecotypes are separated only by ecological barriers.
Conclusions: This is the first demonstration of very early reproductive barriers within a sympatric population of Hymenoptera. It demonstrates that sexual and premating barriers can precede ecological separation. This indicates the complexity of ecotype formation and highlights the general need for more studies within homogenous populations for the identification of the earliest barriers in the speciation process.
Keywords: Ecological speciation; Ecotypes; Inbreeding; Postmating barrier; Premating barrier; Reproductive barriers; Sympatric speciation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Coyne JA, Orr HA. Speciation. Sunderland: Sinauer Associates; 2004.
-
- Butlin R, Debelle A, Kerth C, Snook RR, Beukeboom LW, Castillo RC, et al. What do we need to know about speciation? Trends Ecol Evol. 2012;27:27–39. - PubMed
-
- Otto SP, Whitton J. Polyploid incidence and evolution. Annu Rev Genet. 2000;34:401–437. - PubMed
-
- Mallet J. Hybrid speciation. Nature. 2007;446:279–283. - PubMed
-
- Bird CE, Fernandez-Silva I, Skillings DJ, Toonen RJ. Sympatric speciation in the post “Modern Synthesis” era of evolutionary biology. Evol Biol. 2012;39:158–180.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
