Standard- versus intermediate-dose enoxaparin for anti-factor Xa guided thromboprophylaxis in critically ill patients with COVID-19
- PMID: 34781984
- PMCID: PMC8591599
- DOI: 10.1186/s12959-021-00337-z
Standard- versus intermediate-dose enoxaparin for anti-factor Xa guided thromboprophylaxis in critically ill patients with COVID-19
Abstract
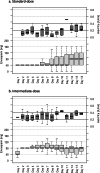
The prevalence of venous thromboembolism (VTE) is high in critically ill patients with COVID-19. Dosing of Low Molecular Weight Heparin (LMWH) for thromboprophylaxis in patients with severe COVID-19 is subject to ongoing debate.In this brief report, we describe our study where we retrospectively examined the efficacy of standard- versus intermediate-dosing of enoxaparin in attaining and maintaining accepted prophylactic levels of anti-Factor Xa (anti-FXa) in critically ill patients with COVID-19.We collected data for all patients with confirmed COVID-19 who were treated with enoxaparin for thromboprophylaxis in a single Intensive Care Unit (ICU) in the United Kingdom between 31st March and 16th November 2020. Standard-dose of enoxaparin was 40 mg subcutaneously once daily for patients with normal renal function and body weight between 50 and 100 kg; the intermediate-dose was 40 mg subcutaneously twice daily. Anti-FXa peak concentrations between 0.2-0.4 IU/ml were considered appropriate for thromboprophylaxis.Age, sex, weight, Body Mass Index, APACHE II score, ICU length of stay, initial P/F ratio and creatinine were not statistically significantly different between standard- and intermediate-dose thromboprophylaxis cohorts. In the standard-dose group, the median initial anti-FXa level was 0.13 (interquartile range 0.06-0.18) compared to 0.26 (0.21-0.33) in the intermediate-dose cohort (p < 0.001). On repeated measurement, in the standard dose cohort, 44 of 95 (46%) anti-FXa levels were < 0.2 IU/ml compared with 24 of 132 (18%) levels in the intermediate-dose cohort even after dose-adjustment. There was one radiologically confirmed pulmonary embolism (PE) on computed tomography pulmonary angiogram during hospital admission in each cohort.Our study supports starting intermediate-dose thromboprophylaxis for critically ill patients with COVID-19 to achieve anti-FXa levels in the accepted thromboprophylactic range although further study is required to investigate whether anti-FXa guided thromboprophylaxis is safe and effective in reducing the incidence of VTEs in critically ill patients with COVID-19.
© 2021. The Author(s).
Conflict of interest statement
None of the authors have declared a competing interest.
Figures
References
-
- FICM. Rapid Update to FICM/ICS Clinical guide for the management of critical care for adults with COVID-19 during the Coronavirus pandemic 2021. https://icmanaesthesiacovid-19.org/covid-cc-guideline-update (accessed 2nd October 2021 2021).
-
- Investigators I. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

